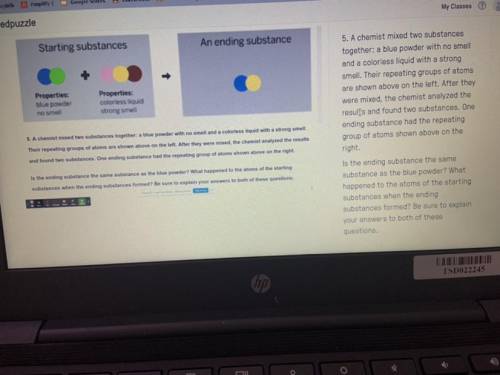
PLEASEEE HELP A chemist mixed two substances
together: a blue powder with no smell
and a col...

PLEASEEE HELP A chemist mixed two substances
together: a blue powder with no smell
and a colorless liquid with a strong
smell. Their repeating groups of atoms
are shown above on the left. After they
were mixed, the chemist analyzed the
results and found two substances. One
ending substance had the repeating
group of atoms shown above on the
right.
Is the ending substance the same
substance as the blue powder? What
happened to the atoms of the starting
substances when the ending
substances formed? Be sure to explain
your answers to both of these

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:40
Achange in the number of neutrons in an atom will change an blank . when the number of protons changes in an atom, a new element will form.
Answers: 2

Chemistry, 22.06.2019 11:00
3) in peaches, [oh]=3.16x10-11 m a) find [h+ ] b) what is the ph? c) is the solution acidic, basic, or neutral?
Answers: 1

Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning calculate the ph of 0.16m ch3cooh which has ka = 1.74 x 10-5 mol dm-3 best answer will be brainliest
Answers: 3

Chemistry, 22.06.2019 17:30
Observation and experimentation have led many scientists to accept a theory about the origin of the universe. this theory is called the big bang theory. scientific evidence collected and observed by scientists around the world suggests that the universe is ever expanding from a hot and dense initial state. what makes this a scientific theory? (2 points)
Answers: 2
You know the right answer?
Questions


Mathematics, 14.06.2021 18:00


Mathematics, 14.06.2021 18:00

Mathematics, 14.06.2021 18:00

Mathematics, 14.06.2021 18:00



English, 14.06.2021 18:00

Biology, 14.06.2021 18:00


Mathematics, 14.06.2021 18:00

Mathematics, 14.06.2021 18:00


Mathematics, 14.06.2021 18:00

Mathematics, 14.06.2021 18:00

Biology, 14.06.2021 18:00

Mathematics, 14.06.2021 18:00

Mathematics, 14.06.2021 18:00





