
Chemistry, 24.04.2021 01:00 kayla114035
Calculate the pH of the solution, given the following (H+). Then identify the 1 point
solution as acidic, basic, or neutral. [H+]=3.98x10^-8*
O 7.4; acidic
7.4;neutral
6.5; neutral
Calculate the pH of the solution, given the following (H+). Then identify the 1 point
solution as acidic, basic, or neutral. [H+]=7.94x10^-5*
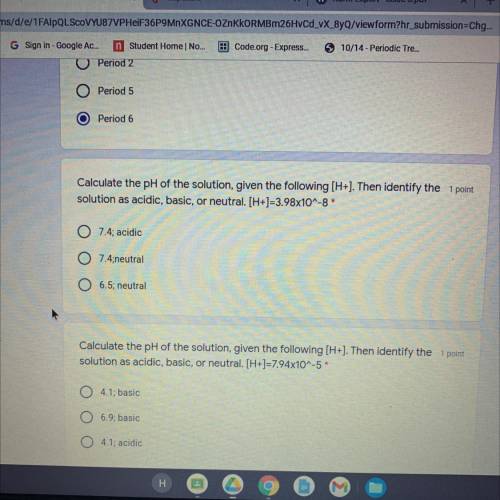

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 12:00
Give the set of reactants (including an alkyl halide and a nucleophile) that could be used to synthesize the following ether: draw the molecules on the canvas by choosing buttons from the tools (for bonds and charges), atoms, and templates toolbars, including charges where needed. ch3ch2och2ch2chch3 | ch3
Answers: 1


Chemistry, 22.06.2019 20:00
Phosphoric acid is a triprotic acid ( =6.9×10−3 , =6.2×10−8 , and =4.8×10−13 ). to find the ph of a buffer composed of h2po−4(aq) and hpo2−4(aq) , which p value should be used in the henderson–hasselbalch equation? p k a1 = 2.16 p k a2 = 7.21 p k a3 = 12.32 calculate the ph of a buffer solution obtained by dissolving 18.0 18.0 g of kh2po4(s) kh 2 po 4 ( s ) and 33.0 33.0 g of na2hpo4(s) na 2 hpo 4 ( s ) in water and then diluting to 1.00 l.
Answers: 3
You know the right answer?
Calculate the pH of the solution, given the following (H+). Then identify the 1 point
solution as...
Questions


Health, 21.01.2022 14:00

Mathematics, 21.01.2022 14:00




Mathematics, 21.01.2022 14:00


Mathematics, 21.01.2022 14:00


History, 21.01.2022 14:00

Chemistry, 21.01.2022 14:00

Advanced Placement (AP), 21.01.2022 14:00

Chemistry, 21.01.2022 14:00

Mathematics, 21.01.2022 14:00


English, 21.01.2022 14:00





