Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:00
Given the following reaction: c3h8+5o2=3co2+4h20 how many grams of co2 will be produced 7 g of c3h8 and 98 g of o2
Answers: 1

Chemistry, 22.06.2019 10:50
A100 kmol/h stream that is 97 mole% carbon tetrachloride (ccl4) and 3% carbon disulfide (cs2) is to be recovered from the bottom of a distillation column. the feed to the column is 16 mole% cs2 and 84% ccl4, and 2% of the ccl4 entering the column is contained in the overhead stream leaving the top of the column. calculate the mass and mole fractions of ccl4 in the overhead stream, and determine the molar flow rates of ccl4 and cs2 in the overhead and feed streams. 12. mw_ccla- 153.82; mw_cs2-76.14.
Answers: 3

Chemistry, 22.06.2019 12:00
Marcel just purchased 1.69 grams of iron fillings in order to make living putty for his 6 year old niece. how many moles of iron are made in his sample?
Answers: 1

Chemistry, 22.06.2019 16:00
Which factor is likely to impact the possible number of compounds ?
Answers: 1
You know the right answer?
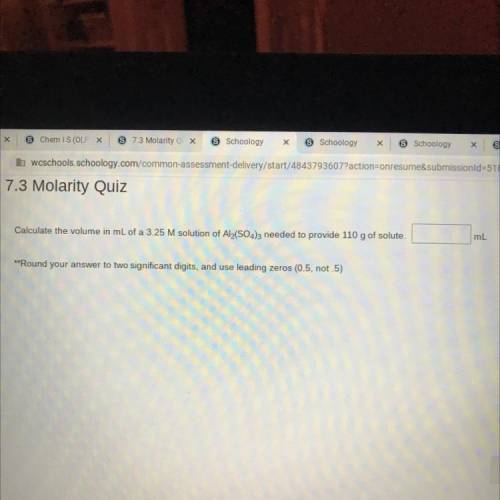
Calculate the volume in mL of a 3.25 M solution of Al2(SO4)3 needed to provide 110 g of solute.
Questions


Chemistry, 09.06.2021 23:50

Mathematics, 09.06.2021 23:50

Mathematics, 09.06.2021 23:50

History, 09.06.2021 23:50


Computers and Technology, 09.06.2021 23:50








Social Studies, 09.06.2021 23:50




History, 09.06.2021 23:50

History, 09.06.2021 23:50