
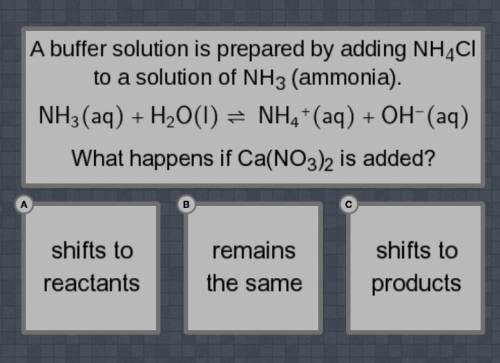
A buffer solution is prepared by adding NH4Cl to a solution of NH3 (ammonia).
NH3(aq) + H2O(I) = NH4+(aq) +OH-(aq0
What happens if Ca(NO3)2 is added?
Shifts to reactants, remain the same, shifts to products.
Please HELP!
I watched the video, but I still don't get it!
I don't know how this works...

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 14:00
650.j is the same amount of energy as? 2720cal1550cal650.cal2.72cal
Answers: 2

Chemistry, 22.06.2019 14:00
Calculate the frequency of a wave in a spring toy. the wave has a speed of 1.1 meters per second and a wavelength of 0.1 meters. *
Answers: 2

Chemistry, 23.06.2019 03:50
Show how to convert the temperature 84.7° c to kelvin. include all steps and label the final answer.
Answers: 1

Chemistry, 23.06.2019 05:50
Which of the following isotopes has the same number of neutrons as phosphorus-31?
Answers: 1
You know the right answer?
A buffer solution is prepared by adding NH4Cl to a solution of NH3 (ammonia).
NH3(aq) + H2O(I) = N...
Questions

Mathematics, 09.10.2021 03:00




Social Studies, 09.10.2021 03:00

Advanced Placement (AP), 09.10.2021 03:00



Biology, 09.10.2021 03:00




Mathematics, 09.10.2021 03:00

Mathematics, 09.10.2021 03:00

Mathematics, 09.10.2021 03:00


Physics, 09.10.2021 03:00

Mathematics, 09.10.2021 03:00


Biology, 09.10.2021 03:00



