
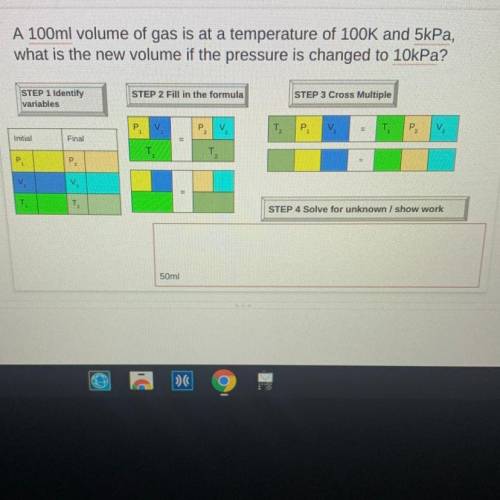
A 100ml volume of gas is at a temperature of 100K and 5kPa,
what is the new volume if the pressure is changed to 10kPa?
STEP 1 Identify
variables
STEP 2 Fill in the formula
STEP 3 Cross Multiple
P
V
T2
P
P2
Initial
Final
P.
P
=
V
V
T
TO
STEP 4 Solve for unknown / show work

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:30
According to periodic trend, which of the following most likely has the highest ionization energy? kr be ni sc
Answers: 3

Chemistry, 22.06.2019 06:00
Ethanol (c2h5oh) is produced from the fermentation of sucrose in the presence of enzymes. c12h22o11(aq) + h2o(g) 4 c2h5oh(l) + 4 co2(g) determine the theoretical yield and the percent yields of ethanol if 680. g sucrose undergoes fermentation and 326.5 g ethanol is obtained. theoretical _ g _ percent %
Answers: 1

Chemistry, 22.06.2019 17:00
According to the kinetic-molecular theory, what happens to a liquid when it is transferred from one container to another? the volume and the shape stay the same. the volume increases to fill the new container, but the shape stays the same. the volume stays the same, but the shape changes to fit the new container. the volume and the shape change to fill the new container.
Answers: 2

Chemistry, 23.06.2019 03:30
In chemistry, the type of an atom (what element it is) is determined by: a) the number of protons it contains in its nucleus.b) the number of neutrons it contains in its nucleus.c) the number of protons it has in a cloud around the nucleus.d) the number of neutrons it has in a cloud around the nucleus.e) the number of electrons it exchanges with its neighbors.
Answers: 1
You know the right answer?
A 100ml volume of gas is at a temperature of 100K and 5kPa,
what is the new volume if the pressure...
Questions

History, 09.04.2021 17:40





Mathematics, 09.04.2021 17:40



Mathematics, 09.04.2021 17:40


World Languages, 09.04.2021 17:40


Mathematics, 09.04.2021 17:40

Advanced Placement (AP), 09.04.2021 17:40



Biology, 09.04.2021 17:40

Chemistry, 09.04.2021 17:40

History, 09.04.2021 17:40

History, 09.04.2021 17:40



