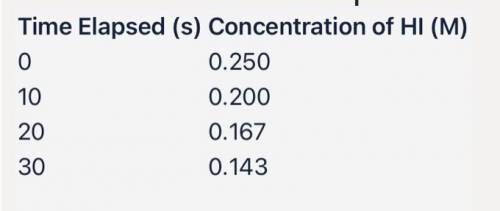
The decomposition of HI(g) is a second order reaction.
2 HI(g) → I2(g) + H2(g)
Above i...

The decomposition of HI(g) is a second order reaction.
2 HI(g) → I2(g) + H2(g)
Above is some data from an experiment in which HI was decomposed.
Using the reaction data above:
(a) If you wanted to create a linear graph for this experiment with time on the x-axis, what variable would you put on the y-axis?
(b) Use the data above to calculate k; include units.
(c) How many seconds will it take for the concentration of HI to reach 0.050 M?
(d) What will be the concentration of HI after 50.0 seconds?
Please I need this ASAP I’m stuck

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Select each correct answer. more than one answer may be correct. which of the following is a characteristic of unicellular organisms? they can possess tissues and organs. all of their functions are performed by a single cell. they are usually microscopic. each of their cells is specialized to perform a specific function.
Answers: 1


Chemistry, 22.06.2019 10:00
A50.0g sample of liquid water at 0.0 c ends up as ice at -20.0 c. how much energy is involved in this change?
Answers: 1

Chemistry, 22.06.2019 11:40
Which of these expressions are correct variations of the combined gas law? p1v1t2 = p2v2t1 both
Answers: 2
You know the right answer?
Questions



Mathematics, 09.09.2021 08:20

History, 09.09.2021 08:20



Mathematics, 09.09.2021 08:20

Mathematics, 09.09.2021 08:20

English, 09.09.2021 08:20

History, 09.09.2021 08:20

History, 09.09.2021 08:20





Mathematics, 09.09.2021 08:20

Mathematics, 09.09.2021 08:20

Chemistry, 09.09.2021 08:20


Mathematics, 09.09.2021 08:20



