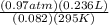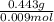Chemistry, 26.04.2021 09:10 kmcgregor4155
An experiment shows that a 236 mL gas sample has a mass of 0.443 g at a pressure of 740 mmHg and a temperature of 22 ∘C. What is the molar mass of the gas?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:30
The table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 3

Chemistry, 22.06.2019 23:00
The data below were determined for the reaction shown below. s2o82– + 3i – (aq) → 2so42– + i3– expt. # [s2o82–] (m) [i –] (m) initial rate 1 0.038 0.060 1.4 × 10 – 5 m/s 2 0.076 0.060 2.8 × 10 – 5 m/s 3 0.076 0.030 1.4 × 10 – 5 m/s the rate law for this reaction must be:
Answers: 1

Chemistry, 23.06.2019 02:00
The bone of a dinosaur and the imprint of a leaf are examples of which kind of fossils? a) index b) body c) amber d) trace
Answers: 1

Chemistry, 23.06.2019 06:30
When microscope slides are stained to show blood cells, the small red blood cells that appear on the slides are much numerous than the large white blood cells. this supports the concept that
Answers: 1
You know the right answer?
An experiment shows that a 236 mL gas sample has a mass of 0.443 g at a pressure of 740 mmHg and a t...
Questions

Biology, 14.12.2021 20:30


Mathematics, 14.12.2021 20:30



English, 14.12.2021 20:30

English, 14.12.2021 20:30

Social Studies, 14.12.2021 20:30

Mathematics, 14.12.2021 20:30

Mathematics, 14.12.2021 20:30


Mathematics, 14.12.2021 20:30



History, 14.12.2021 20:30



History, 14.12.2021 20:30


Geography, 14.12.2021 20:30

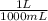 ) = .236 L
) = .236 L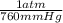 )= 0.97 atm
)= 0.97 atm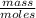 or MM=
or MM= 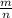 . They're all the same.
. They're all the same.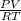 , where R (constant)= 0.082 L atm mol-1 K-1
, where R (constant)= 0.082 L atm mol-1 K-1