
Chemistry, 26.04.2021 14:00 oliviaprejean18
A 9.02-g sample of liquid water at 40.3 °C is heated by the addition of 203.93 J of energy. The final temperature of the water is °C. The specific heat capacity of liquid water is 4.184 J/gK.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:30
Amass of 100.0 g of solute is dissolved in water so that 850. ml of a 0.7500 m solution has been prepared. what is the molar mass of the solute?
Answers: 2

Chemistry, 21.06.2019 22:30
Write the symbol for every chemical element that has atomic number greater than 3 and atomic mass less than 12.0 u.
Answers: 1

Chemistry, 22.06.2019 15:20
Which description best characterizes the motion of particles in a solid?
Answers: 2

Chemistry, 22.06.2019 17:40
How much heat is added if 0.814g of water increase in temperature by 0.351 degree c?
Answers: 3
You know the right answer?
A 9.02-g sample of liquid water at 40.3 °C is heated by the addition of 203.93 J of energy. The fina...
Questions







Mathematics, 23.08.2019 09:30

Social Studies, 23.08.2019 09:30

Mathematics, 23.08.2019 09:30


Biology, 23.08.2019 09:30




History, 23.08.2019 09:30

Mathematics, 23.08.2019 09:30

History, 23.08.2019 09:30


History, 23.08.2019 09:30

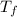 -
- 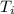
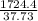 =
= 

