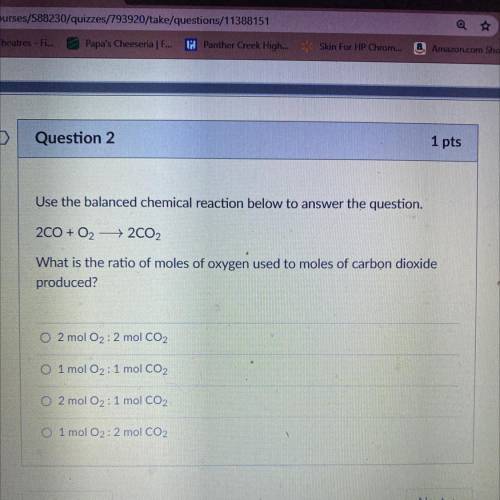
Question 2
Use the balanced chemical reaction below to answer the question.
200 + O2 + 2CO2<...


Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
One does not belong why? ice, gold ,wood ,diamond and table salt
Answers: 1

Chemistry, 22.06.2019 14:30
Calculate the mass of carbon in 97.0 g of sucrose c12h22o11
Answers: 3

Chemistry, 22.06.2019 20:20
Which formula equation represents the burning of sulfur to produce sulfur dioxide? s(s) + o2(g) 4502(9) 2h2s(s) + 302(g) —> 2h20(0) + 2502(9) 4fes2+1102 —> 2fe2o3 + 8502 2802(g) + o2(9) v205 , 2503(9)
Answers: 1

Chemistry, 23.06.2019 01:30
Witch two conditions can limit the usefulness of the kinetic molecular theory in describing gas behavior?
Answers: 2
You know the right answer?
Questions

Computers and Technology, 13.02.2020 05:56



Computers and Technology, 13.02.2020 05:56



History, 13.02.2020 05:56

Mathematics, 13.02.2020 05:56

Physics, 13.02.2020 05:56

Mathematics, 13.02.2020 05:56

Mathematics, 13.02.2020 05:56

History, 13.02.2020 05:56




Mathematics, 13.02.2020 05:56


English, 13.02.2020 05:56