
Chemistry, 26.04.2021 21:10 tamikagoss22
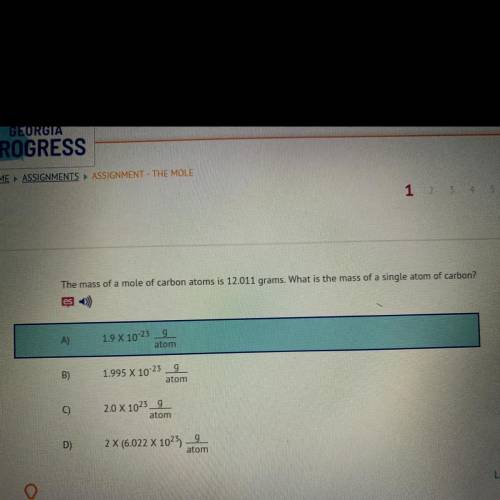
The mass of a mole of carbon atoms is 12.011 grams. What is the mass of a single atom of carbon?
A)9
1.9 X 10-23
atom
B)
g
1.995 X 10-23
atom
C)
2.0 X 1023_9
atom
D)
2 X (6.022 X 1025)
9
atom
Law of Conserv
Y

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 16:30
Acetic acid, hc2h3o2, dissolves and completely dissociates in water and a solvation sphere of water molecules forms around the ions. this solute-solvent interaction
Answers: 1

Chemistry, 21.06.2019 20:30
What was the procedure by which case united states vs lopez went to court
Answers: 1

Chemistry, 22.06.2019 10:40
Which buffer would be better able to hold a steady ph on the addition of strong acid, buffer 1 or buffer 2? explain. buffer 1: a solution containing 0.10 m nh4cl and 1 m nh3. buffer 2: a solution containing 1 m nh4cl and 0.10 m nh3
Answers: 1

Chemistry, 23.06.2019 02:00
Pinene is an unsaturated hydrocarbon found in pine resin. if pinene has m+ = 136 and contains 1 double bond(s) and 2 ring(s); what is its molecular formula? enter the formula in the form ch first, then all other atoms in alphabetical order; do not use subscripts. the formula is case-sensitive
Answers: 3
You know the right answer?
The mass of a mole of carbon atoms is 12.011 grams. What is the mass of a single atom of carbon?
A...
Questions

Business, 09.09.2020 01:01

Mathematics, 09.09.2020 01:01

Biology, 09.09.2020 01:01


Mathematics, 09.09.2020 01:01

Mathematics, 09.09.2020 01:01


Mathematics, 09.09.2020 01:01




Mathematics, 09.09.2020 01:01

Mathematics, 09.09.2020 01:01

Mathematics, 09.09.2020 01:01

Business, 09.09.2020 01:01

Mathematics, 09.09.2020 01:01

Mathematics, 09.09.2020 01:01


Mathematics, 09.09.2020 01:01




