
Chemistry, 26.04.2021 21:30 roperbailey
Calculate the number of moles of iron (III) oxide (Fe 2 O 3 ) produced from 112 L of oxygen
(O 2 ) in the following reaction
4Fe(s) + 3O2 (g) = 2Fe2O3 (s)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:40
Calculate: select the worksheet tab. this tab you calculate the analyte concentration. fill in the first set of boxes ("moles h2so4" and "moles naoh") based on the coefficients in the balanced equation. (if there is no coefficient, the value is 1.) record the appropriate volumes in the "ml naoh" and "ml h2so4" boxes. record the concentration of the titrant in the m naoh box. click calculate. what is the concentration listed
Answers: 2

Chemistry, 22.06.2019 14:30
What type(s) of intermolecular forces are expected between ch3ch2cooh molecules? dipole forces, induced dipole forces, hydrogen bonding
Answers: 1

Chemistry, 22.06.2019 16:00
How do dying stars contribute to the formation of planets
Answers: 1

Chemistry, 22.06.2019 22:10
What is the indicator of the number of ions in solution? the amount of conductivity the amount of precipitate the amount of solute added
Answers: 1
You know the right answer?
Calculate the number of moles of iron (III) oxide (Fe 2 O 3 ) produced from 112 L of oxygen
(O 2 )...
Questions

Mathematics, 13.10.2020 01:01

Mathematics, 13.10.2020 01:01

Mathematics, 13.10.2020 01:01

Mathematics, 13.10.2020 01:01




Mathematics, 13.10.2020 01:01



History, 13.10.2020 01:01




Mathematics, 13.10.2020 01:01

Social Studies, 13.10.2020 01:01



Mathematics, 13.10.2020 01:01

Mathematics, 13.10.2020 01:01

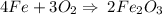

 (Since one mole at STP = 22.4 L)
(Since one mole at STP = 22.4 L)


