
Chemistry, 26.04.2021 23:40 lilpeepxliltracy
calculate the amount of heat in joules needed to raise the temperature of 25 g of silver by 100°
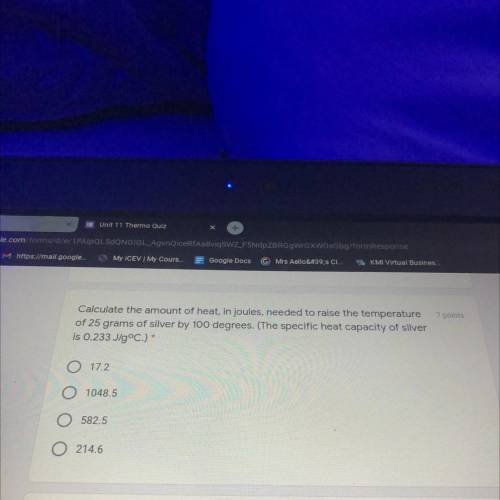

Answers: 1


Another question on Chemistry

Chemistry, 23.06.2019 00:00
Which is true about metals used for jewelry, such as platinum and gold? a. they have low flammability. b. they have low reactivity. c. they have high flammability. d. they have high reactivity.
Answers: 1

Chemistry, 23.06.2019 04:10
Which of the following is described by the equation h2o(s)+ heat=h2o(i) a freezing melting condensing evaporating
Answers: 2

Chemistry, 23.06.2019 11:00
Which of the following reactions represents an exothermic reaction? nh3(g) + 12.0 kcal ½n2(g) + 3/2 h2(g) ch4 + 2o2 co2 + 2h2o + 212,800 cal c + 2s cs2, h = 27,550 cal c(graphite) c(diamond), h = 0.45 kcal 2h2o 2h2 + o2, h = +58 kcal
Answers: 1

Chemistry, 23.06.2019 13:30
Consider this reaction taking place in a closed 2 liter container: 2so2(g) + o2(g) → 2so3(g) if the volume of the container is decreased to 1 liter, what will happen to the equilibrium of the reaction? it will shift left. it will shift right. it will remain constant it will decrease by half
Answers: 3
You know the right answer?
calculate the amount of heat in joules needed to raise the temperature of 25 g of silver by 100°
Questions

Social Studies, 15.04.2021 22:40

Mathematics, 15.04.2021 22:40

Mathematics, 15.04.2021 22:40

Social Studies, 15.04.2021 22:40

Physics, 15.04.2021 22:40


Mathematics, 15.04.2021 22:40


English, 15.04.2021 22:40



Mathematics, 15.04.2021 22:40

Mathematics, 15.04.2021 22:40


Physics, 15.04.2021 22:40

Social Studies, 15.04.2021 22:40

Computers and Technology, 15.04.2021 22:50

Health, 15.04.2021 22:50




