Please help
The thermochemical equation for the combustion of propane gas is:
CH4 (g)...

Chemistry, 27.04.2021 02:10 coolgirl5679
Please help
The thermochemical equation for the combustion of propane gas is:
CH4 (g) + 2O2 (g) CO2 (g) + 2H2O (I), ΔH = -890 kJ/mol
Calculate much heat is released when 3.5 moles of propane have a combustion reaction.
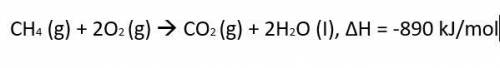

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:00
Chen drew a diagram to compare the ways in which different organisms obtain nitrogen. which label belongs to the area marked z?
Answers: 3



Chemistry, 22.06.2019 22:30
Which process describes vaporization that takes place below the surface of a liquid? condensation melting boiling evaporation
Answers: 1
You know the right answer?
Questions

Computers and Technology, 17.03.2021 23:40

Mathematics, 17.03.2021 23:40



Mathematics, 17.03.2021 23:40

Mathematics, 17.03.2021 23:40

Mathematics, 17.03.2021 23:40


Mathematics, 17.03.2021 23:40

History, 17.03.2021 23:40


English, 17.03.2021 23:40

Mathematics, 17.03.2021 23:40



English, 17.03.2021 23:40

Mathematics, 17.03.2021 23:40

Mathematics, 17.03.2021 23:40

English, 17.03.2021 23:40




