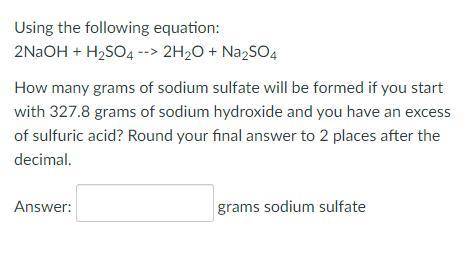
Using the following equation:
2NaOH + H2SO4 --> 2H2O + Na2SO4
How many grams of sod...

Chemistry, 27.04.2021 18:20 Dashavu4626
Using the following equation:
2NaOH + H2SO4 --> 2H2O + Na2SO4
How many grams of sodium sulfate will be formed if you start with 327.8 grams of sodium hydroxide and you have an excess of sulfuric acid? Round your final answer to 2 places after the decimal.
_ grams sodium sulfate

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Which compound contains both ionic and covalent bonds? a) hbr b)cbr4 c)nabr d) naoh
Answers: 2

Chemistry, 22.06.2019 09:00
The nuclear fission process releases neutrons and question 27 options: alpha particles electrons energy beta particles
Answers: 1

Chemistry, 22.06.2019 18:00
Many pharmaceutical drugs are organic compounds that were originally synthesized in the laboratory. which two scientific disciplines are bridged by pharmaceutical drugs? chemistry and forensics chemistry and medicine biology and forensics biology and criminology
Answers: 2

Chemistry, 23.06.2019 01:00
If a sample of radioactive isotopes takes 600 minutes to decay from 400 grams to 50 grams, what is the half-life of the isotope?
Answers: 1
You know the right answer?
Questions





Mathematics, 17.07.2020 02:01



Mathematics, 17.07.2020 02:01


Mathematics, 17.07.2020 02:01


Business, 17.07.2020 02:01


Chemistry, 17.07.2020 02:01





Mathematics, 17.07.2020 02:01

Chemistry, 17.07.2020 02:01



