
Chemistry, 27.04.2021 18:50 ajfijeoinf2750
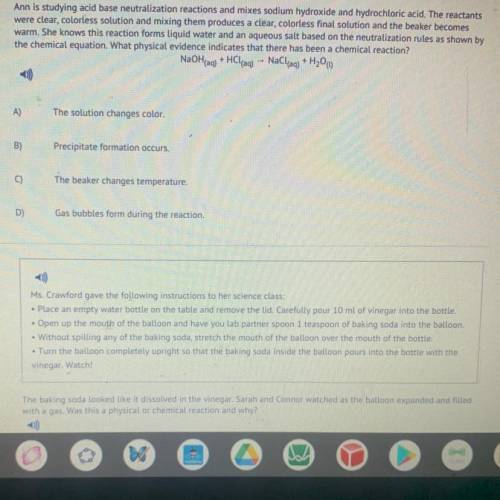
Ann is studying acid base neutralization reactions and mixes sodium hydroxide and hydrochloric acid. The reactants were clear, colorless solution and mixing them produces a clear, colorless final solution and the beaker becomes warm. She knows this reaction forms liquid water and an aqueous salt based on the neutralization rules as shown by the chemical equationWhat physical evidence indicates that there has been a chemical reaction NaOH (2q) +HCl (2q) NaCl \ 2Q) +H 2 O (l) A) The solution changes color. B ) Precipitate formation occurs. The beaker changes temperature . D) Gas bubbles form during the reaction.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:10
When electrolyzing copper (ll) chloride, what reaction takes place at the anode? what reaction takes place at the cathode?
Answers: 1

Chemistry, 22.06.2019 23:30
Why do oxygen have a strong attractive force for electrons
Answers: 2

Chemistry, 23.06.2019 00:10
Covalent compounds: mastery test select the correct answer what is formed when atoms join together with a covalent bond? a. an ion b. a molecule c. a neutral atom d. a noble gas
Answers: 3

Chemistry, 23.06.2019 03:30
Why do electrons further from the nucleus have more energy
Answers: 1
You know the right answer?
Ann is studying acid base neutralization reactions and mixes sodium hydroxide and hydrochloric acid....
Questions


English, 01.08.2019 05:50





Mathematics, 01.08.2019 05:50


Mathematics, 01.08.2019 05:50

History, 01.08.2019 05:50

Mathematics, 01.08.2019 05:50

Mathematics, 01.08.2019 05:50

Mathematics, 01.08.2019 05:50

History, 01.08.2019 06:00

Chemistry, 01.08.2019 06:00


Mathematics, 01.08.2019 06:00


History, 01.08.2019 06:00




