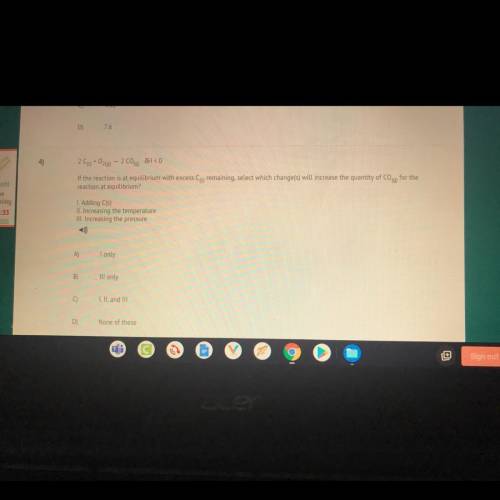
4)
209+0260) - 2 C00) JH < 0
If the reaction is at equilibrium with excess Cs remaining,...

Chemistry, 28.04.2021 02:50 mahagonylabeyta
4)
209+0260) - 2 C00) JH < 0
If the reaction is at equilibrium with excess Cs remaining, select which change(s) will increase the quantity of Coq) for the
reaction at equilibrium?
I. Adding C(s)
II. Increasing the temperature
III. Increasing the pressure

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 13:30
1) which of the following is the best example of a physical change? a) sugar dissolving in tea b) firefly glowing 2) in the combustion of ethane, what is/are the reactants? c2h6 + o2 ==> co2 + h2o a) c2h6 and o2 b) co2 and c2h6
Answers: 2

Chemistry, 22.06.2019 22:00
4.25g sample of solid ammonium nitrate dissolves in 60.0g of water in a coffee-cup calorimeter, the temperature drops from 22.0 c to 16.9 c. assume that the specific heat of the solution is the same as that of pure water. calculate delta(h) (in kj/mol nh4no3) for the solution proces.
Answers: 2

Chemistry, 22.06.2019 23:30
If maltose undergoes hydrolysis what subunits does it results to?
Answers: 2

Chemistry, 23.06.2019 05:30
Awhite powder is added to a solution. the images show observations made before the powder is added, just after the powder has been added, and a little while later. (the liquid in the small beaker is phenol red solution.) what evidence shows that a chemical change has taken place?
Answers: 1
You know the right answer?
Questions

Biology, 13.07.2019 20:00


Biology, 13.07.2019 20:00


Arts, 13.07.2019 20:00


History, 13.07.2019 20:00

History, 13.07.2019 20:00

Chemistry, 13.07.2019 20:00

History, 13.07.2019 20:00

English, 13.07.2019 20:00



History, 13.07.2019 20:00


Biology, 13.07.2019 20:00






