
Chemistry, 28.04.2021 03:00 donaji1024perez
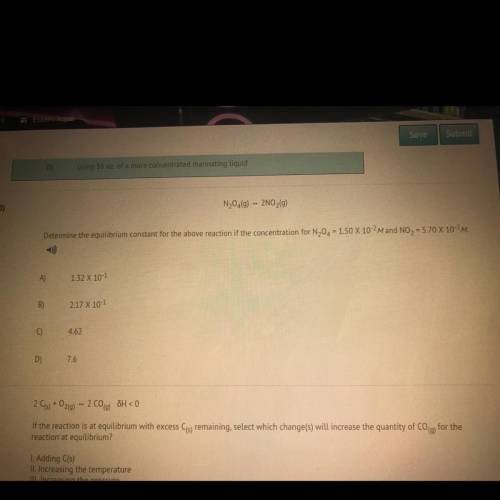
Determine the equilibrium constant for the above reaction if the concentration for N20 = 150 X 10-2M and NO2 = 5.70 X 10-2M.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:30
In an oxidation-reduction reaction, oxidation is what happens when a reactant
Answers: 1


Chemistry, 22.06.2019 19:00
Avolleyball player hit a ball with a mass of 0.25 kg. the average acceleration of the ball is 15.5 m/s². how much force did the volleyball player apply to the ball? 62.0 n 3.87 n 62.0 m/s² 3.87 m/s²
Answers: 2

Chemistry, 23.06.2019 03:00
Determine type of reaction & predict the product c3h12+o2 =
Answers: 1
You know the right answer?
Determine the equilibrium constant for the above reaction if the concentration for N20 = 150 X 10-2M...
Questions

English, 06.01.2021 06:30


Mathematics, 06.01.2021 06:30

Spanish, 06.01.2021 06:30


Mathematics, 06.01.2021 06:30



Social Studies, 06.01.2021 06:30


Mathematics, 06.01.2021 06:30



Chemistry, 06.01.2021 06:30

Mathematics, 06.01.2021 06:30

English, 06.01.2021 06:30


Spanish, 06.01.2021 06:30

Mathematics, 06.01.2021 06:30

Mathematics, 06.01.2021 06:30



