Answers: 3


Another question on Chemistry



Chemistry, 23.06.2019 13:00
What type of reaction is this equation c2h5s + o2 > co2 + h2o + so2
Answers: 2

Chemistry, 23.06.2019 15:30
Consider these four line graphs representing speed in meters/second, where each x-axis is labelled in seconds and each y-axis is labelled in meters. which line graph indicates the greatest speed at 5 seconds?
Answers: 1
You know the right answer?
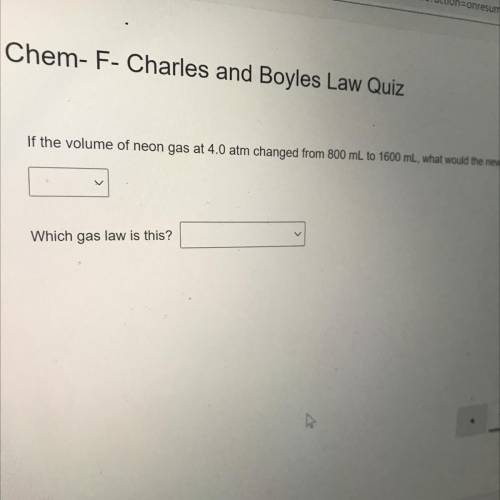
If the volume of neon gas at 4.0 atm changed from 800 mL to 1600 mL, what would the new pressure be?...
Questions




Mathematics, 07.04.2020 02:26




Social Studies, 07.04.2020 02:27

Mathematics, 07.04.2020 02:27

Mathematics, 07.04.2020 02:27

Mathematics, 07.04.2020 02:27

English, 07.04.2020 02:27


Biology, 07.04.2020 02:27



Social Studies, 07.04.2020 02:27


History, 07.04.2020 02:27