1)
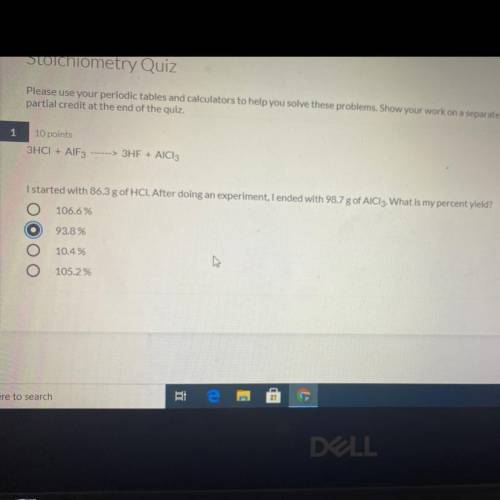
3HCI + AlF3---> 3HF + AICI:
Please help ASAP!!!
I started with 86.3 g of H...

Chemistry, 28.04.2021 19:20 enchantednights
1)
3HCI + AlF3---> 3HF + AICI:
Please help ASAP!!!
I started with 86.3 g of HCI. After doing an experiment, I ended with 98.7 g of AlCl3. What is my percent yield?
A) 106.6 %
B) 93.8 %
C) 10.4%
D) 105.2 %

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:30
When you perform this reaction, what could remain at the end of the reaction? check all that apply. excess reactant aqueous copper chloride excess reactant aluminum oxygen product solid copper carbon dioxide product aqueous aluminum chloride water
Answers: 2

Chemistry, 22.06.2019 06:00
24. a sports ball is inflated to an internal pressure of 1.85 atm at room temperature (25 °c). if the ball is then played with outside where the temperature is 7.5 °c, what will be the new pressure of the ball? assume the ball does not change in volume nor does any air leak from the ball a) 0.555 atm b) 1.74 atm c) 1.85 atm d) 1.97 atm
Answers: 2

Chemistry, 22.06.2019 06:30
If 1.8 l of water is added to 2.5l of a 7.0 molarity koh solution, what is the molarity of the new solution
Answers: 1

Chemistry, 22.06.2019 18:10
Measurements that have similar values are: a. usually accurate b. sometimes accurate c. always accurate d. never accurate
Answers: 1
You know the right answer?
Questions









Mathematics, 24.04.2020 18:00

Social Studies, 24.04.2020 18:00





Spanish, 24.04.2020 18:00

Biology, 24.04.2020 18:00



Mathematics, 24.04.2020 18:00

Mathematics, 24.04.2020 18:00



