Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 20:00
What is the molarity of the solution produced when 145 g of nacl is dissolved in sufficient water to prepare 2.75 l of solution?
Answers: 1

Chemistry, 23.06.2019 00:10
Apropane torch is lit inside a hot air balloon during preflight preparations to inflate the balloon. which condition of the gas remains constant
Answers: 2

Chemistry, 23.06.2019 02:30
Which of the four hypothetical substances you investigated would be most harmful to living organisms? 50 points!
Answers: 2

Chemistry, 23.06.2019 08:00
What is the temperature in kelvin of a gas if it is allowed to expand from 1.50 l to 4.50 l? the initial temperature is 10.0°c and pressure is constant throughout the change. which equation should you use? t2= v2/v1 t1 what is the final temperature? ⇒ 849 k these are the answers.
Answers: 1
You know the right answer?
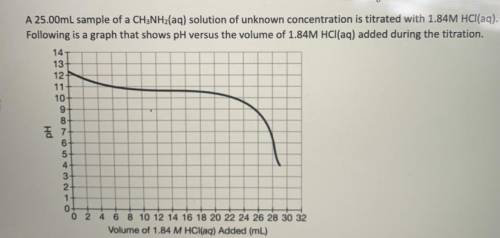
If 28.25mL of 1.84M HCl(aq) was required to reach the equivalence point, calculate the
concentrati...
Questions


History, 15.04.2020 00:45



Biology, 15.04.2020 00:46

Advanced Placement (AP), 15.04.2020 00:46











English, 15.04.2020 00:46


Computers and Technology, 15.04.2020 00:46