A.
Cu(OH)2 +
HCI →
H2O + __ CuCl2 (2 points)
-
b. If 3.00 moles of Cu(OH),...

Chemistry, 28.04.2021 20:30 amanuelwold
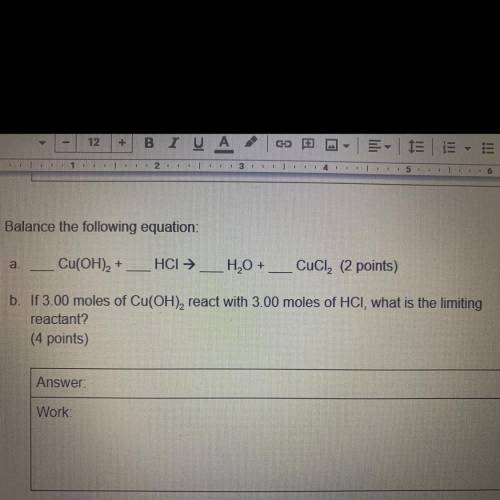
A.
Cu(OH)2 +
HCI →
H2O + __ CuCl2 (2 points)
-
b. If 3.00 moles of Cu(OH), react with 3.00 moles of HCI, what is the limiting
reactant?
(4 points)

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Describe the particles of all three phases of matter in the root beer float. (how are the particles arranged and moving? )
Answers: 3

Chemistry, 21.06.2019 23:00
Achef makes salad dressing by mixing oil, vinegar, and spices, as shown. which type of matter is the salad dressing?
Answers: 1

Chemistry, 22.06.2019 05:00
Given sno2 + 2h2 - sn + 2h20 tin oxide reacts with hydrogen to produce tin and water. how many moles of sno2 are needed to produce 500.0 grams of sn?
Answers: 3

Chemistry, 22.06.2019 05:30
What is the mass of each element in a 324.8 sample of co2
Answers: 1
You know the right answer?
Questions

Computers and Technology, 29.03.2021 19:30


Mathematics, 29.03.2021 19:30

English, 29.03.2021 19:30


History, 29.03.2021 19:30



Mathematics, 29.03.2021 19:30


Mathematics, 29.03.2021 19:30

Mathematics, 29.03.2021 19:30

Chemistry, 29.03.2021 19:30

Health, 29.03.2021 19:30

Mathematics, 29.03.2021 19:30


Mathematics, 29.03.2021 19:30


Biology, 29.03.2021 19:30

Engineering, 29.03.2021 19:30



