
Chemistry, 28.04.2021 20:40 gigimasters71p7tc6l
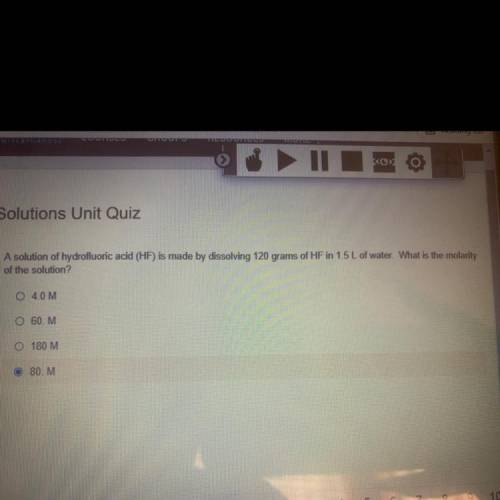
A solution of hydrofluoric acid (HF) is made by dissolving 120 grams of HF in 1.5 L of water. What is the molarity
of the solution?
HELP ASAP!!!

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:00
When a spring is compressed, the energy changes from kinetic to potential. which best describes what is causing this change?
Answers: 3

Chemistry, 22.06.2019 10:00
Part 1: include important facts found through your research. part 2: include your visual display. include your summary of “the chemistry of water” from the national science foundation website. include your experiment. part 3: include responses to the reflection questions.
Answers: 1

Chemistry, 22.06.2019 12:20
Consider the reaction of a(g) + b(g) + c(g) => d(g) for which the following data were obtained: experiment initial [a], mol/l initial [b], mol/l initial [c], mol/l initial rate, mol/l.s 1 0.0500 0.0500 0.0100 6.25 x 10^-3 2 0.100 0.0500 0.0100 2.50 x 10^-2 3 0.100 0.100 0.0100 1.00 x 10^-1 4 0.0500 0.0500 0.0200 6.25 x 10^-3 what is the rate law for the reaction?
Answers: 3

You know the right answer?
A solution of hydrofluoric acid (HF) is made by dissolving 120 grams of HF in 1.5 L of water. What i...
Questions


Mathematics, 16.07.2019 08:40

Physics, 16.07.2019 08:40





English, 16.07.2019 08:40


Mathematics, 16.07.2019 08:40


History, 16.07.2019 08:40



Mathematics, 16.07.2019 08:40

History, 16.07.2019 08:40

Mathematics, 16.07.2019 08:40

Health, 16.07.2019 08:40

English, 16.07.2019 08:40



