
Chemistry, 28.04.2021 22:50 kodiebclay
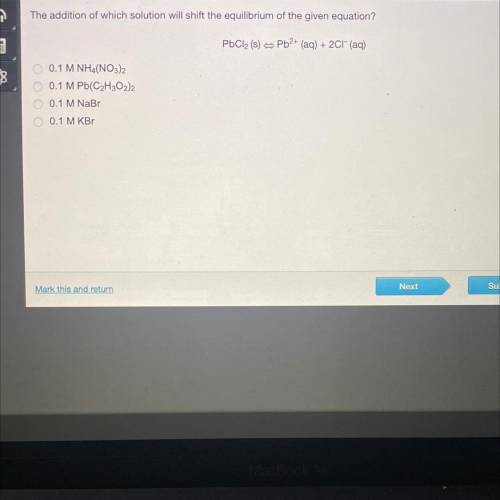
The addition of which solution will shift the equilibrium of the given equation?
PbCl2 (s) = Pb2+ (aq) + 2Cl(aq)
0 0.1 M NHA(NO3)2
0.1 M Pb(C2H302)2
0.1 M NaBr
0.1 M KB
What’s the answer?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Aroller coaster car is traveling down a track at 22 m/s. the car has a mass of 2000 kg. what is the kinetic energy of the car? a) 22,000 j b) 968,000 j c) 484,000 j d) 44,000 j
Answers: 2

Chemistry, 22.06.2019 11:40
Calculate the number of kilojoules to warm 125 g of iron from 23.5°c to 78.0°c.
Answers: 3

Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning calculate the ph of 0.16m ch3cooh which has ka = 1.74 x 10-5 mol dm-3 best answer will be brainliest
Answers: 3

Chemistry, 22.06.2019 17:30
Energy defines the different "states" of matter. in no more than 3 sentences, describe the amount of kinetic energy that each of the 3 states of matter possesses and relate that to the atom/molecular motion of each "state".
Answers: 2
You know the right answer?
The addition of which solution will shift the equilibrium of the given equation?
PbCl2 (s) = Pb2+...
Questions



Advanced Placement (AP), 30.01.2020 20:59

Social Studies, 30.01.2020 20:59



History, 30.01.2020 20:59


Social Studies, 30.01.2020 20:59

Biology, 30.01.2020 20:59



English, 30.01.2020 20:59

English, 30.01.2020 20:59

Biology, 30.01.2020 20:59




Mathematics, 30.01.2020 20:59

Mathematics, 30.01.2020 20:59



