
Chemistry, 29.04.2021 19:10 niceguy1997
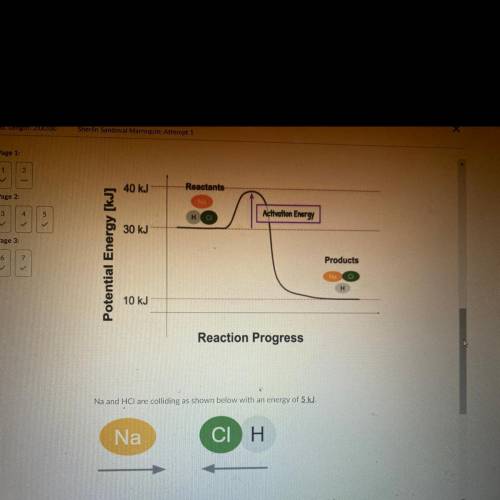
Na and HCl are colliding as shown below with an energy
Q1. Will this collision be effective? Explain
Q2. What are 2 things you could change to increase the rate of this reaction? Use
the following words to explain your number of collision, and kinetic energy.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:00
About 70 percent of the earth's surface is water-covered, and about 96.5 percent of all earth's water is salt water. identify the watery feature on earth that is made of freshwater rather than salt water. a) bay b) glacier c) ocean d) sea it is not incomplete this is the true question
Answers: 1

Chemistry, 22.06.2019 11:00
An object becomes electrically charged when: electrons are created in it electrons from it are destroyed electrons are transferred to it protons from it are destroyed protons are created in it
Answers: 1

Chemistry, 22.06.2019 13:30
In a ni-cd battery, a fully charged cell is composed of nickelic hydroxide. nickel is an element that has multiple oxidation states. assume the following proportions of the states: nickel charge proportions found 0 0.17 +2 0.3 +3 0.33 +4 0.5 (a) determine the mean of the nickel charge. enter the answer to 2 decimal places.(b) determine the cumulative distribution function of nickel charge.
Answers: 2

Chemistry, 23.06.2019 00:30
What is bromine+calcium iodide--> calcium bromide +iodine balanced
Answers: 1
You know the right answer?
Na and HCl are colliding as shown below with an energy
Q1. Will this collision be effective? Expla...
Questions


Mathematics, 24.03.2020 03:33





Mathematics, 24.03.2020 03:33

Geography, 24.03.2020 03:33


Computers and Technology, 24.03.2020 03:33


Mathematics, 24.03.2020 03:33

Mathematics, 24.03.2020 03:33


History, 24.03.2020 03:33


Mathematics, 24.03.2020 03:33






