Question:
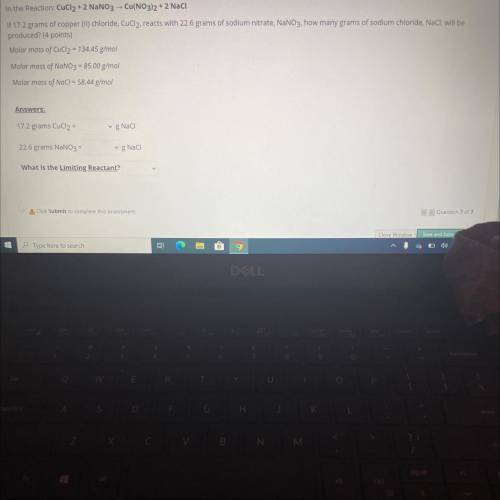
In the Reaction: CuCl2 + 2 NaNO3 -- Cu(NO3)2 + 2 Naci
If 17.2 grams of copper (II)...

Chemistry, 29.04.2021 19:40 quickestlearner8562
Question:
In the Reaction: CuCl2 + 2 NaNO3 -- Cu(NO3)2 + 2 Naci
If 17.2 grams of copper (II) chloride, CuCl2, reacts with 22.6 grams of sodium nitrate, NaNO3. how many grams of sodium chloride, NaCl, will be
produced? (4 points)
Molar mass of CuCl2 = 134.45 g/mol
Molar mass of NaNO3 = 85.00 g/mol
Molar mass of NaCl = 58.44 g/mol
Answers:
17.2 grams CuCl2 =
gNaci
v
22.6 grams NaNO3 =
g Naci
What is the Limiting Reactant?
No

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:00
The reactions shown here can be combined to make the overall reaction c(s) + h2o(g) ⇌ co(g) + h2(g) by reversing some and/or dividing all the coefficients by a number. a. c(s) + o2(g) → co2(g) k=1.363×10^69 b. 2 h2(g) + o2(g) → 2 h2o(g) k=1.389×10^80 c. 2co(g) + o2 (g) → 2 co2(g) k=1.477×10^90
Answers: 1

Chemistry, 22.06.2019 13:30
If the concentration of phosphate in the cytosol is 2.0 mm and the concentration of phosphate in the surrounding fluid is 0.1 mm, how could the cell increase the concentration of phosphate in the cytosol? a) passive transportb) diffusionc) active transportd) osmosise) facilitated diffusion
Answers: 3

Chemistry, 22.06.2019 14:30
Need ! asap will mark 10 pts using the room temperature line (orange line) and your periodic table, make lists that identify the state of matter (gas, liquid, or solid) in which each element you plotted exists at room temperature. explain your answers
Answers: 1

Chemistry, 22.06.2019 20:30
Consider the following unbalanced equation for the combustion of hexane: αc6h14(g)+βo2(g)→γco2(g)+δh2o(g) part a balance the equation. give your answer as an ordered set of numbers α, β, γ, use the least possible integers for the coefficients. α α , β, γ, δ = nothing request answer part b determine how many moles of o2 are required to react completely with 5.6 moles c6h14. express your answer using two significant figures. n n = nothing mol request answer provide feedback
Answers: 2
You know the right answer?
Questions



Mathematics, 16.10.2020 18:01






Mathematics, 16.10.2020 18:01


Mathematics, 16.10.2020 18:01


Mathematics, 16.10.2020 18:01



Physics, 16.10.2020 18:01

Mathematics, 16.10.2020 18:01

Geography, 16.10.2020 18:01


Mathematics, 16.10.2020 18:01



