
Chemistry, 29.04.2021 22:20 amortegaa805
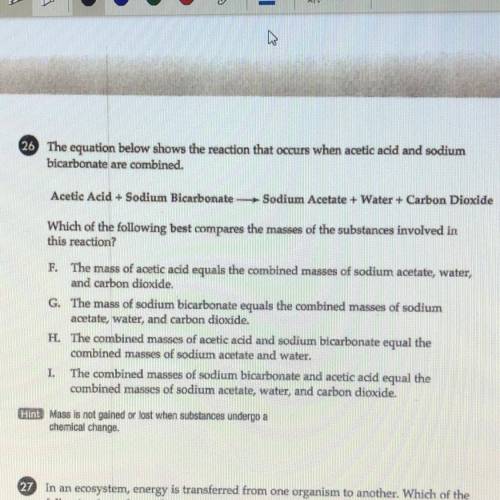
WILL MARK BRAINLIEST PLZ HURRY. 26 The equation below shows the reaction that occurs when acetic acid and sodium
bicarbonate are combined.
Acetic Acid + Sodium Bicarbonate Sodium Acetate + Water + Carbon Dioxide
Which of the following best compares the masses of the substances involved in
this reaction?
F. The mass of acetic acid equals the combined masses of sodium acetate, water,
and carbon dioxide.
G. The mass of sodium bicarbonate equals the combined masses of sodium
acetate, water, and carbon dioxide.
H. The combined masses of acetic acid and sodium bicarbonate equal the
combined masses of sodium acetate and water.
I. The combined masses of sodium bicarbonate and acetic acid equal the
combined masses of sodium acetate, water, and carbon dioxide.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Using the periodic table, complete the table to describe each atom. type in your answers
Answers: 3

Chemistry, 22.06.2019 06:00
If you burn 10 kilograms of wood in a fire (combustion) what is the weight of the products after the fire has finished burning the wood?
Answers: 3

Chemistry, 22.06.2019 15:10
Which statement describes the phase change that occurs when dry ice is placed in an open container at room temperature?
Answers: 1

You know the right answer?
WILL MARK BRAINLIEST PLZ HURRY. 26 The equation below shows the reaction that occurs when acetic aci...
Questions

Spanish, 25.04.2020 00:48

Mathematics, 25.04.2020 00:48


Mathematics, 25.04.2020 00:48

Mathematics, 25.04.2020 00:48




History, 25.04.2020 00:48

Mathematics, 25.04.2020 00:48




Computers and Technology, 25.04.2020 00:48




Social Studies, 25.04.2020 00:49

Health, 25.04.2020 00:49

Engineering, 25.04.2020 00:49



