
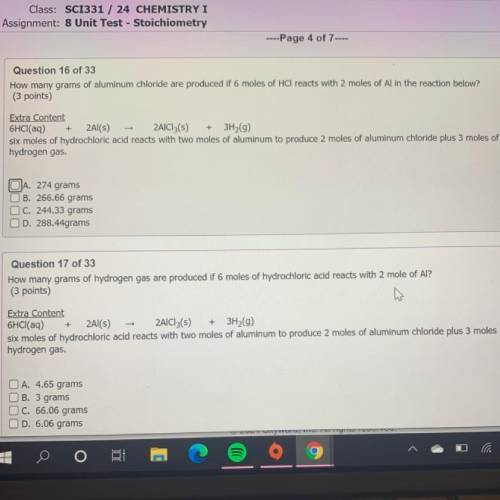
How many grams of aluminum chloride are produced if 6 moles of HCl reacts with 2 moles of Al in the reaction below?
(3 points)
Extra Content
6HCl(aq) + 2Al(s) - 2AlCl3(s) + 3H2(9)
six moles of hydrochloric acid reacts with two moles of aluminum to produce 2 moles of aluminum chloride plus 3 moles of
hydrogen gas.
JA. 274 grams
B. 266.66 grams
C. 244.33 grams
D. 288.44grams

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:30
Given that the molar mass of nano3 is 85.00 g/mol, what mass of nano3 is needed to make 4.50 l of a 1.50 m nano3solution? use .6.75 g18.9 g255 g574 g
Answers: 1

Chemistry, 22.06.2019 01:30
100 points answer quick the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1

Chemistry, 22.06.2019 07:00
If there is any 12 to 14 girls that need a boyfriend just follow me and let me know
Answers: 1

Chemistry, 22.06.2019 17:10
In which block of the periodic table is uranium (u) found? s blockd blockp blockf block
Answers: 1
You know the right answer?
How many grams of aluminum chloride are produced if 6 moles of HCl reacts with 2 moles of Al in the...
Questions


Physics, 08.07.2019 17:40

Physics, 08.07.2019 17:40

Physics, 08.07.2019 17:40

Physics, 08.07.2019 17:40


Social Studies, 08.07.2019 17:40

History, 08.07.2019 17:40


Biology, 08.07.2019 17:40

Social Studies, 08.07.2019 17:40

Biology, 08.07.2019 17:40


History, 08.07.2019 17:40

Biology, 08.07.2019 17:40

History, 08.07.2019 17:40

Chemistry, 08.07.2019 17:40

Mathematics, 08.07.2019 17:40


History, 08.07.2019 17:40



