Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:40
Astudent is given a sample of a blue copper sulfate hydrate. he weighs the sample in a dry covered porcelain crucible and got a mass of 23.875 g for the crucible, lid, and sample. the mass of the empty crucible and lid was found earlier to be 22.652 g. he then heats the crucible to expel the water of hydration, keeping the crucible at red heat for 10 minutes with the lid slightly ajar. on colling, he finds the mass of crucible, lid, and contents to be 23.403 g. the sample was changed in the process to very light clue anhydrous cuso4. if there are again 100.0 g of hydrate, how many grams of cuso4 are in it? how many moles of cuso4? (hint: molar mass of cuso4 = 159.6 g / mole. what per cent of the hydrate is cuso4? you may convert the mass of cuso4 to moles.)
Answers: 3


Chemistry, 22.06.2019 09:30
Mike and mitchell decide to have a foot race. they mark off a stretch of 100 yards, and recruit cindy to work the stopwatch. after running the race and looking at the results, cindy declared that mitchell was the fastest. so how did the boys times compare?
Answers: 3

Chemistry, 22.06.2019 09:30
Which element is the least metallic between cadmium, silver, zinc, or iron?
Answers: 1
You know the right answer?
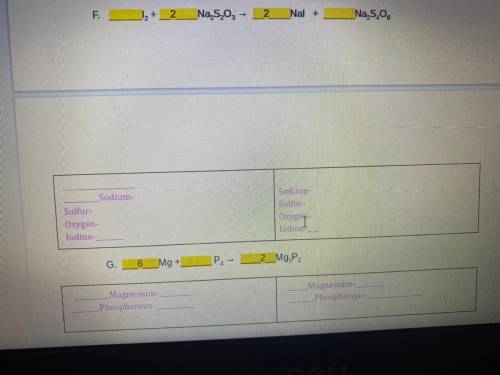
PLEASE A REAL PERSON THIS IS DUE AT 12 AM PLS DONT IGNORE :(
...
...
Questions


Mathematics, 11.07.2019 02:00

Geography, 11.07.2019 02:00

Mathematics, 11.07.2019 02:00

Physics, 11.07.2019 02:00



Mathematics, 11.07.2019 02:00

Mathematics, 11.07.2019 02:00

Biology, 11.07.2019 02:00

Biology, 11.07.2019 02:00


Mathematics, 11.07.2019 02:00

Mathematics, 11.07.2019 02:00


Health, 11.07.2019 02:00


English, 11.07.2019 02:00