
Chemistry, 30.04.2021 14:00 hannahmckain
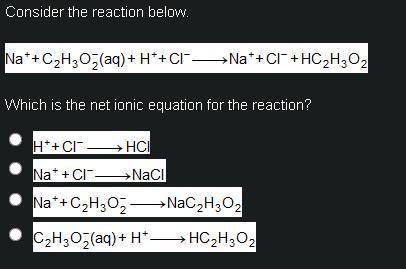
Consider the reaction below.
Upper N a superscript plus, plus upper C subscript 2 upper H subscript 3 upper O subscript 2 superscript minus (a q) plus upper H superscript plus, plus upper C l superscript minus right arrow upper N a superscript plus, plus upper C l superscript minus plus upper H upper subscript 2 upper H subscript 3 upper O subscript 2.
Which is the net ionic equation for the reaction?
Upper H superscript plus, plus upper C l superscript minus right arrow upper H upper C l.
Upper N a superscript plus, plus upper C l superscript minus right arrow upper N a upper C l.
Upper N a superscript plus, plus upper C subscript 2 upper H subscript 3 upper o subscript 2 superscript minus (a q) plus upper H superscript plus, right arrow upper N a upper C subscript 2 upper H subscript 3 upper O subscript 2.
Upper C subscript 2 upper h subscript 3 upper O subscript 2 superscript minus (a q) plus upper H superscript plus right arrow upper H upper C subscript 2 upper H subscript 3 upper O subscript 2.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Two nitro no2 groups are chemically bonded to a patch of surface. they can't move to another location on the surface, but they can rotate (see sketch at right). it turns out that the amount of rotational kinetic energy each no2 group can have is required to be a multiple of ε, where =ε×1.010−24 j. in other words, each no2 group could have ε of rotational kinetic energy, or 2ε, or 3ε, and so forth — but it cannot have just any old amount of rotational kinetic energy. suppose the total rotational kinetic energy in this system is initially known to be 32ε. then, some heat is removed from the system, and the total rotational kinetic energy falls to 18ε. calculate the change in entropy. round your answer to 3 significant digits, and be sure it has the correct unit symbol.
Answers: 2

Chemistry, 22.06.2019 07:30
Given that 1 mi = 1760 yd, determine what conver- sion factor is appropriate to convert 1849 yd to miles; to convert 2.781 mi to yards.
Answers: 2

Chemistry, 22.06.2019 09:40
Sulfur dioxide and oxygen react to form sulfur trioxide during one of the key steps in sulfuric acid synthesis. an industrial chemist studying this reaction fills a 25.0l tank with 4.5 mol of sulfur dioxide gas and 4.5 mol of oxygen gas at 30.°c. he then raises the temperature, and when the mixture has come to equilibrium measures the amount of sulfur trioxide gas to be 1.4 mol. calculate the concentration equilibrium constant for the reaction of sulfur dioxide and oxygen at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 3

Chemistry, 22.06.2019 15:00
Large helium-filled balloons are used to lift scientific equipment to high altitudes. what is the pressure inside such a balloon if it starts out at sea level with a temperature of 10.0ºc and rises to an altitude where its volume is twenty times the original volume and its temperature is – 50.0ºc ?
Answers: 2
You know the right answer?
Consider the reaction below.
Upper N a superscript plus, plus upper C subscript 2 upper H subscrip...
Questions

English, 24.01.2022 23:50

Mathematics, 24.01.2022 23:50

Chemistry, 24.01.2022 23:50

Geography, 24.01.2022 23:50



Mathematics, 24.01.2022 23:50


Physics, 24.01.2022 23:50

Computers and Technology, 24.01.2022 23:50

Mathematics, 24.01.2022 23:50



Mathematics, 24.01.2022 23:50

Mathematics, 24.01.2022 23:50

Mathematics, 24.01.2022 23:50






