
Chemistry, 30.04.2021 16:30 woodfordmaliky
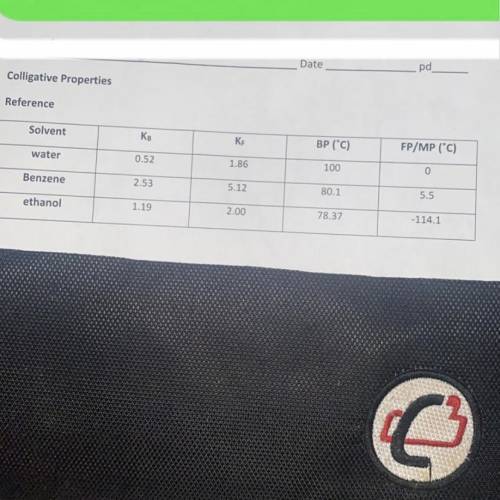
When 14.8 grams of an ionic substance ( cation + anion have the same charge ) is dissolved in 125.0 g of ethanol , the solution begins to boil at 81.2C Calculate the molar mass of this solute. (i need work aswell)

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 15:00
1) how many electrons are in each energy level of the following elements? a. he b. na c. na d. ne 2) how many valence electrons are percent in the following atoms? a. s b. mg c. be d. cl 3) which of the following elements are stable as atoms? a. he b. o c. cl d. ar if you are able to provide the work as to how you got the answers that would be greatly appreciated. : )
Answers: 1

Chemistry, 21.06.2019 20:30
1. calculate the approximate enthalpy of the reaction in joules. estimate that 1.0 ml of vinegar has the same thermal mass as 1.0 ml of water. iqnore the thermal mass of th sodium bicarbonate. note: it takes about 4.2 joules () to change 1.0 gram (1.0ml) of water 1.0 c
Answers: 2

Chemistry, 22.06.2019 03:30
At a temperature of 393 k, the temperature of a sample of nitrogen is 1.07 atm what will the pressure be at a temperature of 478 k
Answers: 1

Chemistry, 22.06.2019 10:00
The tendency of water molecules to stick together is referred to as a) adhesion b) polarity c) cohesion d) transpiration e) evaporation
Answers: 1
You know the right answer?
When 14.8 grams of an ionic substance ( cation + anion have the same charge ) is dissolved in 125.0...
Questions


Computers and Technology, 01.09.2019 18:00




Health, 01.09.2019 18:00


History, 01.09.2019 18:00

Mathematics, 01.09.2019 18:00

History, 01.09.2019 18:00

Physics, 01.09.2019 18:00



Mathematics, 01.09.2019 18:00




English, 01.09.2019 18:00

Arts, 01.09.2019 18:00

Mathematics, 01.09.2019 18:00



