
Chemistry, 30.04.2021 17:30 janinecastillo01
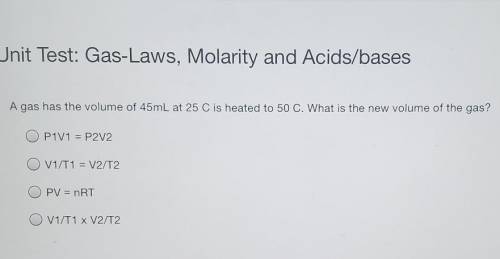
A gas has the volume of 45mL at 25 C is heated to 50 C. What is the new volume of the gas? P1V1 = P2V2 V1/T1 = V2/T2 PV = nRT V1/T1 x V2/T2

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
200. ml of 3.00 m nacl solution is diluted to a final volume of 500. ml. what is the molarity of the final solution?
Answers: 2

Chemistry, 22.06.2019 13:30
If the concentration of phosphate in the cytosol is 2.0 mm and the concentration of phosphate in the surrounding fluid is 0.1 mm, how could the cell increase the concentration of phosphate in the cytosol? a) passive transportb) diffusionc) active transportd) osmosise) facilitated diffusion
Answers: 3

Chemistry, 22.06.2019 19:00
Mercury metal is poured into a graduated cylinder that holds exactly 22.5 ml the mercury used to fill the cylinder mass in 306.0 g from this information calculate the density of mercury
Answers: 2

Chemistry, 22.06.2019 19:30
Phosphorous can form an ion called phosphide, which has the formula p3−. this ion can form an ion called phosphide, which has the formula p3−. this ion properties very similar to those of pforms when a phosphorus atom loses three protonsis called a cationcontains 18 electrons
Answers: 2
You know the right answer?
A gas has the volume of 45mL at 25 C is heated to 50 C. What is the new volume of the gas? P1V1 = P2...
Questions

Mathematics, 14.01.2021 04:20

Mathematics, 14.01.2021 04:20

Mathematics, 14.01.2021 04:20

History, 14.01.2021 04:20

Mathematics, 14.01.2021 04:20


English, 14.01.2021 04:20

Mathematics, 14.01.2021 04:20

History, 14.01.2021 04:20

Mathematics, 14.01.2021 04:20





Chemistry, 14.01.2021 04:20

English, 14.01.2021 04:20

History, 14.01.2021 04:20


Mathematics, 14.01.2021 04:20



