Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 11:00
Which statement correctly identifies the scientific question and describes why the question is scientific? question 1 refers to the supernatural.question 2 reflects a moral or social value.question 3 refers to something that can be measured.question 4 reflects a question that can’t be observed.
Answers: 1

Chemistry, 22.06.2019 19:10
Δu of , in kj/kg, as it isto k, (a)as a of , (b) at , (c) at .
Answers: 2

Chemistry, 23.06.2019 00:30
What is bromine+calcium iodide--> calcium bromide +iodine balanced
Answers: 1

Chemistry, 23.06.2019 05:40
Why is it incorrect to balance a chemical equation by changing the subscripts? explain.
Answers: 2
You know the right answer?
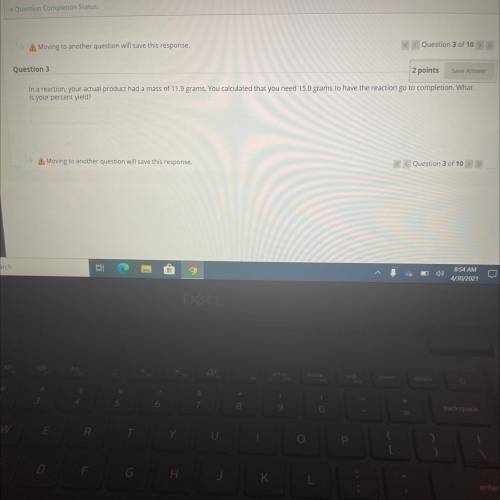
In a reaction, your actual product had a mass of 11.9 grams. You calculated that you need 15.0 grams...
Questions

Mathematics, 14.04.2020 02:22



Mathematics, 14.04.2020 02:22





Mathematics, 14.04.2020 02:22

Mathematics, 14.04.2020 02:22

Physics, 14.04.2020 02:22

English, 14.04.2020 02:22

Mathematics, 14.04.2020 02:22


History, 14.04.2020 02:22


Mathematics, 14.04.2020 02:23

Computers and Technology, 14.04.2020 02:23

Mathematics, 14.04.2020 02:23