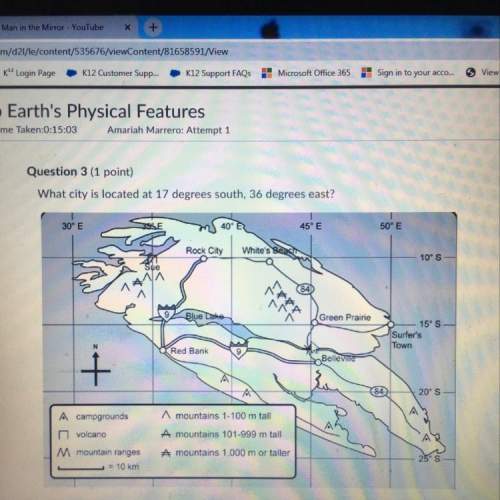
Chemistry, 30.04.2021 22:30 lhecker007
A student placed a small piece of magnesium ribbon in a conical flask and added
60 mL hydrochloric acid. The student observed that when the hydrochloric acid
came into contact with the magnesium ribbon, bubbles formed indicating the
release of hydrogen gas. The magnesium dissolved and magnesium chloride
solution formed. Based on the student's observations, which type of reaction
occurred?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:50
Compare the equilibrium constants for the systems shown in the table. which favors products the most? which favors products the least? rank these systems in order from most to least in terms of favoring products rather than reactants. d > b > a > c c > a > b > d b > c > d > a a > d > c > b
Answers: 1

Chemistry, 22.06.2019 11:00
Predict the products of the following acid-base reactions, and predict whether the equilibrium lies to the left or to the right of the reaction arrow.part ao2-(aq)+h2o(l)< => express your answer as part of a chemical equation. identify all of the phases in your answer.o2-(aq)+h2o(l) < => oh-(aq)+oh-(aq)part bpredict whether the equilibrium lies to the left or to the right of the equation in previous part.h2o is a stronger acid than oh–, so the equilibrium lies to the right.h2o is a weaker acid than oh–, so the equilibrium lies to the left.h2o is a stronger acid than oh–, so the equilibrium lies to the left.h2o is a weaker acid than oh–, so the equilibrium lies to the right.part cch3cooh(aq)+hs? (aq) < => express your answer as part of a chemical equation. identify all of the phases in your answer.ch3cooh(aq)+hs-(aq) < => h2s(aq)+c2h3o2-(aq)h2s(aq)+c2h3o2-(aq)part dpredict whether the equilibrium lies to the left or to the right of the equation in previous part.ch3cooh is a weaker acid than h2s, so the equilibrium lies to the right.ch3cooh is a weaker acid than h2s, so the equilibrium lies to the left.ch3cooh is a stronger acid than h2s, so the equilibrium lies to the right.ch3cooh is a stronger acid than h2s, so the equilibrium lies to the left.part eno2-(aq)+h2o(l) < => express your answer as part of a chemical equation. identify all of the phases in your answer.no2-(aq)+h2o(l) < => part fpredict whether the equilibrium lies to the left or to the right of the equation in previous part.hno2 is a stronger acid than h2o, so the equilibrium lies to the right.hno2 is a weaker acid than h2o, so the equilibrium lies to the left.hno2 is a stronger acid than h2o, so the equilibrium lies to the left.hno2 is a weaker acid than h2o, so the equilibrium lies to the right.
Answers: 1

Chemistry, 22.06.2019 15:00
Why does a plastic bottle that is sealed at a high altitude change it’s shape when taken to lower altitude
Answers: 2

Chemistry, 23.06.2019 05:30
Elizabeth has two separate samples of the same substance. sample is in the liquid state, and the other is in the solid state. the two samples most likely differ in which property?
Answers: 1
You know the right answer?
A student placed a small piece of magnesium ribbon in a conical flask and added
60 mL hydrochloric...
Questions

Mathematics, 06.05.2020 19:01

Computers and Technology, 06.05.2020 19:01



English, 06.05.2020 19:01

Biology, 06.05.2020 19:01

Mathematics, 06.05.2020 19:01



English, 06.05.2020 19:01


Advanced Placement (AP), 06.05.2020 19:01


Mathematics, 06.05.2020 19:01

Geography, 06.05.2020 19:01


Health, 06.05.2020 19:01

Mathematics, 06.05.2020 19:01

Chemistry, 06.05.2020 19:01