
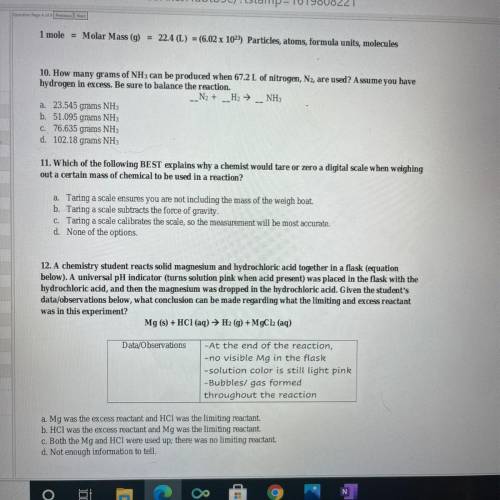
10. How many grams of NH 3 can be produced when 67.2 L of nitrogen, , are used? Assume you have N 2 hydrogen in excess. Be sure to balance the reaction. 12. A chemistry student reacts solid magnesium and hydrochloric acid together in a flask (equation below). A universal pH indicator (turns solution pink when acid present) was placed in the flask with the hydrochloric acid, and then the magnesium was dropped in the hydrochloric acid. Given the student's data/ observations below, what conclusion can be made regarding what the limiting and excess reactant was in this experiment?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 15:00
Which theory was contradicted by experiments with the photoelectric effect? light spreads out after it passes through a small opening. as soon as light strikes metal, electrons will be ejected. visible light, regardless of color, will cause the ejection of electrons when striking metal. the kinetic energy of ejected electrons depends on the frequency of light that strikes the metal.
Answers: 2

Chemistry, 22.06.2019 20:20
Which symbol can be used to indicate the pressure at which a chemical reaction is carried out? 25°c 2 atm pa
Answers: 2


Chemistry, 23.06.2019 10:30
Which of the following pairs of elements is most likely to form an ionic compound? a oxygen and fluorine b sodium and aluminum c calcium and chlorine d nitrogen and sulfur
Answers: 1
You know the right answer?
10. How many grams of NH 3 can be produced when 67.2 L of nitrogen, , are used? Assume you have N 2...
Questions




Mathematics, 21.01.2022 07:00

English, 21.01.2022 07:00


Mathematics, 21.01.2022 07:10



Social Studies, 21.01.2022 07:10





Mathematics, 21.01.2022 07:10

Mathematics, 21.01.2022 07:10


Mathematics, 21.01.2022 07:10

Biology, 21.01.2022 07:10

Biology, 21.01.2022 07:10



