
Chemistry, 01.05.2021 08:00 jakeyywashere
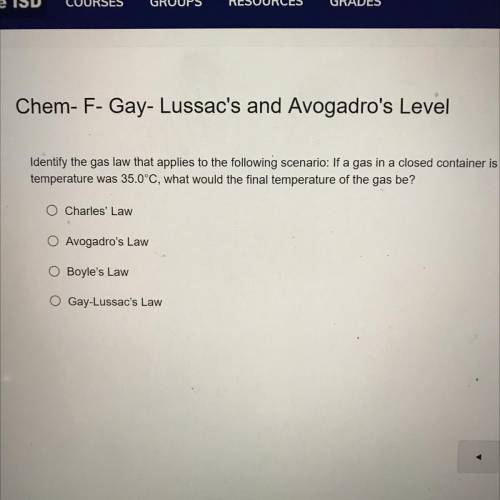
Identify the gas law that applies to the following scenario: If a gas in a closed container is pressurized from 18.0 atm to 14.0 atm and its original
temperature was 35.0°C, what would the final temperature of the gas be?
Charles' Law
O Avogadro's Law
O Boyle's Law
O Gay-Lussac's Law

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 16:30
How does decreasing the gas volume affect the pressure of a gas?
Answers: 1

Chemistry, 21.06.2019 17:30
Aspirin has a density of 1.40 g/cm3 what is the volume in cubic centimeters of a tablet weighing 320 mg ?
Answers: 1

Chemistry, 21.06.2019 21:30
It takes 945.kj/mol to break a nitrogen-nitrogen triple bond. calculate the maximum wavelength of light for which a nitrogen-nitrogen triple bond could be broken by absorbing a single photon.
Answers: 3

Chemistry, 22.06.2019 10:30
Rocks, as they are compressed, begin forming mountains above the earth's surface when two continental plates converge. the continental crust increases in depth as the mountains grow above. the himalayan mountains formed at a convergent plate boundary in this manner. the rocks are smashed together causing them to due to the intense heat and pressure from the colliding plates and eventually forming rock. a) melt; igneous b) layer; sedimentary c) recrystallize; metamorphic d) melt into the earth's interior; metamorphic
Answers: 1
You know the right answer?
Identify the gas law that applies to the following scenario: If a gas in a closed container is press...
Questions

Mathematics, 10.10.2019 05:30

Mathematics, 10.10.2019 05:30

Arts, 10.10.2019 05:30

Mathematics, 10.10.2019 05:30

Mathematics, 10.10.2019 05:30



Mathematics, 10.10.2019 05:30



Mathematics, 10.10.2019 05:30

History, 10.10.2019 05:30

English, 10.10.2019 05:30


Computers and Technology, 10.10.2019 05:30


Health, 10.10.2019 05:30

Mathematics, 10.10.2019 05:30

Biology, 10.10.2019 05:30

History, 10.10.2019 05:30




