What is the mass of each of the following?
(a) 5.34 x 10-2 mol CuCl2
(b) 1.50 X 102 mol Ca(O...

Chemistry, 02.05.2021 18:40 kutemigos9211
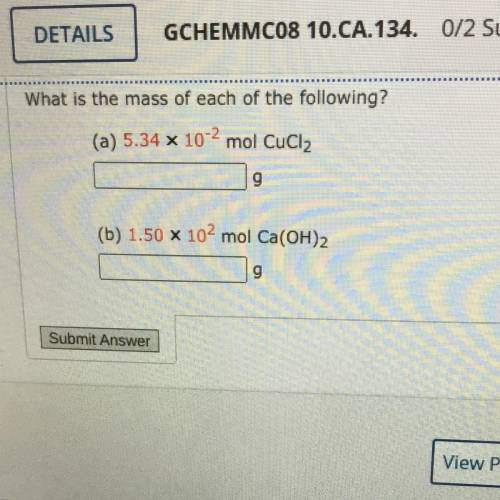
What is the mass of each of the following?
(a) 5.34 x 10-2 mol CuCl2
(b) 1.50 X 102 mol Ca(OH)2

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:30
Draw the skeletal structures of two different molecules that are each made of 5 carbon atoms and 12 hydrogen atoms.
Answers: 1

Chemistry, 22.06.2019 12:30
Suppose you wanted to make 100 grams of water. what is the molar mass of water (h2o)?
Answers: 2

Chemistry, 23.06.2019 00:30
What is the percent by mass of magnesium sulfate in mgso4.7h2o
Answers: 3

Chemistry, 23.06.2019 04:50
The diagin dilutepage 6 of 12a6a5(a)fluorine, chlorine, bromine and iodine are placed in the same group of theperiodic table.state the common name used to describe elements in this group.(i)state the group in which the elements are placed and explain whythey are placed in that group.(ii)which of the above named elements is a solid at roomtemperature and pressure?
Answers: 2
You know the right answer?
Questions

Mathematics, 09.05.2021 14:00

English, 09.05.2021 14:00

Mathematics, 09.05.2021 14:00

Computers and Technology, 09.05.2021 14:00

Mathematics, 09.05.2021 14:00

Mathematics, 09.05.2021 14:00

French, 09.05.2021 14:00

Social Studies, 09.05.2021 14:00

Mathematics, 09.05.2021 14:00


Mathematics, 09.05.2021 14:00

Mathematics, 09.05.2021 14:00




Mathematics, 09.05.2021 14:00

Social Studies, 09.05.2021 14:00





