2. For the reaction
NH3 + O2 - NO + H2O
a. Balance the equation.
b. What is the...

Chemistry, 03.05.2021 01:00 aaronnnn6998
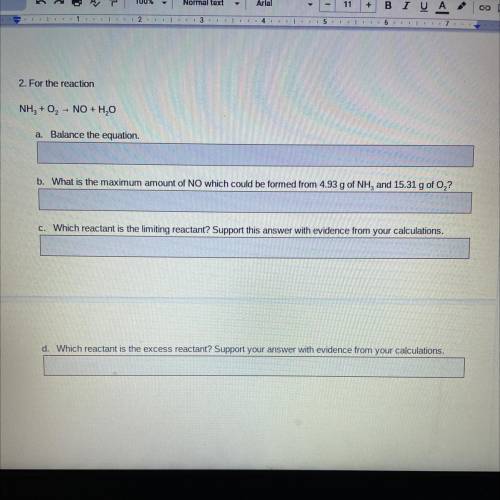
2. For the reaction
NH3 + O2 - NO + H2O
a. Balance the equation.
b. What is the maximum amount of NO which could be formed from 4.93 g of NH, and 15.31 g of O,?
C. Which reactant is the limiting reactant? Support this answer with evidence from your calculations.
d. Which reactant is the excess reactant? Support your answer with evidence from your calculations.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Asolution contains 225 g of sodium chloride, nacl, dissolved in enough water to make a 0.25 l of solution. what is the molarity of the solution?
Answers: 2


Chemistry, 23.06.2019 02:30
Which words or phrases identify layers of groundwater? check all that apply. water table kettle lake saturation zone underground lake sinkhole will give brainiest, answer quickly.
Answers: 1

Chemistry, 23.06.2019 08:00
Can anyone answer these questions? ? i need it before 1: 00pm today
Answers: 1
You know the right answer?
Questions





Mathematics, 21.01.2021 14:00



History, 21.01.2021 14:00






Mathematics, 21.01.2021 14:00

Biology, 21.01.2021 14:00

Mathematics, 21.01.2021 14:00

Mathematics, 21.01.2021 14:00

Mathematics, 21.01.2021 14:00

Business, 21.01.2021 14:00




