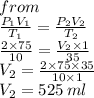Chemistry, 03.05.2021 18:30 katherine3084
3. At 10.0°C and 2.00 atm, the volume of the gas is 75.0 mL. If the temperature of the gas increases to
35.0°C and the pressure decreases to 1.00 atm, what is the new volume?
a. 1630 mL
b. 138 ml
c. 163 mL
d. 0.0017mL

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:50
Using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 pb+2(aq) + 2cl -(aq). if the value of ksp was determined to be only 1.2 x 10-2: too much solid has dissolved. additional precipitate is forming. the solution is unsaturated. the ions are now combining to reduce their concentrations.
Answers: 3

Chemistry, 22.06.2019 09:30
Which element is the least metallic between cadmium, silver, zinc, or iron?
Answers: 1

Chemistry, 22.06.2019 20:30
Select all the correct answers.which compounds have the empirical formula ch20? (multiple answers)a.c2h4o2b.c3h603c.ch2o2d.c5h1005e.c6h1206
Answers: 2

Chemistry, 23.06.2019 05:00
Match each term to its description. match term definition excess reactant a) reactant that can produce a lesser amount of the product limiting reactant b) amount of product predicted to be produced by the given reactants theoretical yield c) reactant that can produce more of the product
Answers: 3
You know the right answer?
3. At 10.0°C and 2.00 atm, the volume of the gas is 75.0 mL. If the temperature of the gas increases...
Questions

Business, 04.08.2019 00:00

Business, 04.08.2019 00:00

History, 04.08.2019 00:00

Computers and Technology, 04.08.2019 00:00

Computers and Technology, 04.08.2019 00:00





Computers and Technology, 04.08.2019 00:00

History, 04.08.2019 00:00


Chemistry, 04.08.2019 00:00

English, 04.08.2019 00:00

Spanish, 04.08.2019 00:10


Mathematics, 04.08.2019 00:10