
Chemistry, 03.05.2021 20:10 kevonmajor
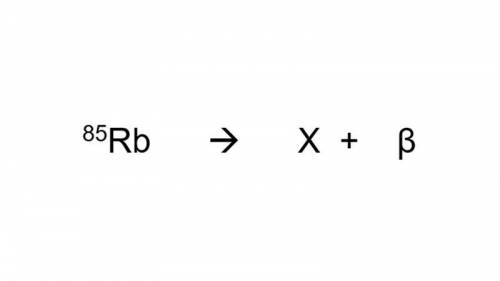
Reações Nucleares: O isótopo do elemento Rubídio de número de massa 85 sofre decaimento segundo a equação a seguir. O número atômico do isótopo que X representa é igual a:
*obs: Verifique o número atômico do Rb na Tabela Periódica*
a-) 38
b-) 36
c-) 86
d-) 84
a-) 37

Answers: 2


Another question on Chemistry

Chemistry, 23.06.2019 00:00
Mercury turns to a vapor at 629.88 k. how much heat is lost when 75.0 g of mercury vapor at 650 k condenses to a liquid at 297 k?
Answers: 1

Chemistry, 23.06.2019 00:20
Steam reforming of methane ( ch4) produces "synthesis gas," a mixture of carbon monoxide gas and hydrogen gas, which is the starting point for many important industrial chemical syntheses. an industrial chemist studying this reaction fills a 1.5 l flask with 3.5 atm of methane gas and 1.3 atm of water vapor at 43.0°c. he then raises the temperature, and when the mixture has come to equilibrium measures the partial pressure of carbon monoxide gas to be 1 .0 atm. calculate the pressure equilibrium constant for the steam reforming of methane at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 1

Chemistry, 23.06.2019 03:30
If you need to add 27.50ml of a solution, which piece of glassware would you use to deliver this volume and explain how you would determine if the 27.50 ml was measured?
Answers: 2

Chemistry, 23.06.2019 03:30
If 2 molecules of one reactant combine with 3 molecules of another to produce 5 molecules of a product, then what is the representation of the reaction?
Answers: 1
You know the right answer?
Reações Nucleares: O isótopo do elemento Rubídio de número de massa 85 sofre decaimento segundo a eq...
Questions


English, 07.04.2021 19:40

Mathematics, 07.04.2021 19:40

English, 07.04.2021 19:40



Mathematics, 07.04.2021 19:40

Mathematics, 07.04.2021 19:40

Mathematics, 07.04.2021 19:40

Chemistry, 07.04.2021 19:40


Mathematics, 07.04.2021 19:40



Mathematics, 07.04.2021 19:40


Mathematics, 07.04.2021 19:40





