
Chemistry, 03.05.2021 20:30 glstephens04
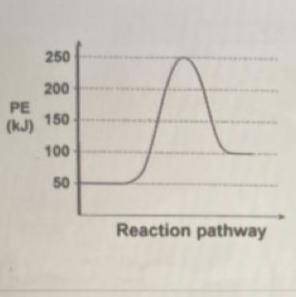
The reaction energy diagram below shows the energy change for a reaction as it proceeds from left to right. The starting energy of the reactants is shown on the left side and the final energy of the products is shown on the right side.
What is the Activation Energy for this reaction?
A. The activation energy would be lower
B. The activation energy would be higher
C. The final energy of the products would be higher
D. The final energy of the products would be lower

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:00
6what is the importance of water on earth? a) it keeps the top layer of the geosphere cool b) it allows life to exist c) it provides ice at the poles d) it creates earth's blue color from space
Answers: 2

Chemistry, 22.06.2019 08:00
Will give ! what are the advantages and disadvantages of nuclear power? check all that apply. one advantage of nuclear energy is that it does not produce carbon dioxide emissions. storage of nuclear waste is a short-term problem associated with nuclear energy. the problem with uranium mining is that a large quantity of uranium must be extracted to meet energy needs because the energy release from uranium fission is so low. safe operation of a nuclear power plant can be jeopardized by a human mistake.
Answers: 1

Chemistry, 22.06.2019 10:40
If an area has high air pressure and low humidity, what type of weather will it most likely have? plz !
Answers: 1

You know the right answer?
The reaction energy diagram below shows the energy change for a reaction as it proceeds from left to...
Questions

Mathematics, 09.09.2021 05:40

History, 09.09.2021 05:40



Mathematics, 09.09.2021 05:40

Mathematics, 09.09.2021 05:40

Mathematics, 09.09.2021 05:40

Mathematics, 09.09.2021 05:40

Advanced Placement (AP), 09.09.2021 05:40


Business, 09.09.2021 05:40

Biology, 09.09.2021 05:40


Mathematics, 09.09.2021 05:40

Mathematics, 09.09.2021 05:40


Computers and Technology, 09.09.2021 05:40



History, 09.09.2021 05:40



