
Chemistry, 03.05.2021 21:10 estefaniapenalo
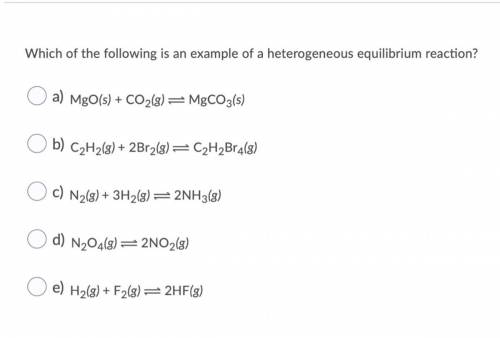
Which of the following is an example of a heterogeneous equilibrium reaction? O a) MgO(s) + CO2(g) = MgCO3(s) O b) C2H2(g) + 2Br2(g) = C2H2Br4(8) O c) N2(g) + 3H2(g) = 2NH3(8) O d) N204(8) =2NO2(8) O e) H2(g) + F2(8)=2HF(3)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:30
This active feature of earth's crust in building mountain ranges as well as islands. this feature is a a) cavern. b) earthquake. c) mountain. d) volcano.
Answers: 2

Chemistry, 22.06.2019 06:00
Ethanol (c2h5oh) is produced from the fermentation of sucrose in the presence of enzymes. c12h22o11(aq) + h2o(g) 4 c2h5oh(l) + 4 co2(g) determine the theoretical yield and the percent yields of ethanol if 680. g sucrose undergoes fermentation and 326.5 g ethanol is obtained. theoretical _ g _ percent %
Answers: 1


Chemistry, 22.06.2019 18:10
Measurements that have similar values are: a. usually accurate b. sometimes accurate c. always accurate d. never accurate
Answers: 1
You know the right answer?
Which of the following is an example of a heterogeneous equilibrium reaction? O a) MgO(s) + CO2(g) =...
Questions



Mathematics, 22.07.2019 20:30


English, 22.07.2019 20:30

Mathematics, 22.07.2019 20:30


Mathematics, 22.07.2019 20:30

Chemistry, 22.07.2019 20:30

Mathematics, 22.07.2019 20:30



Mathematics, 22.07.2019 20:30

Physics, 22.07.2019 20:30

Mathematics, 22.07.2019 20:30

Mathematics, 22.07.2019 20:30

Mathematics, 22.07.2019 20:30

Chemistry, 22.07.2019 20:30




