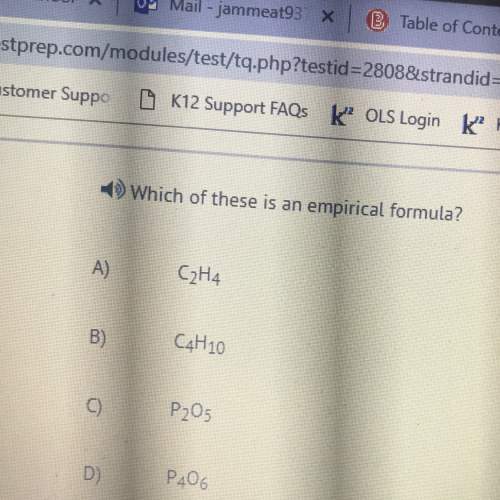
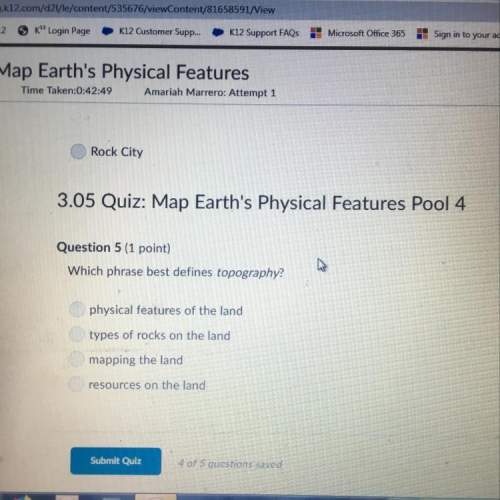
Chemistry, 04.05.2021 09:20 kartchnercamryn10
Consider the titration of a 33.0 mL sample of 0.180 M HBr with 0.205 M KOH. Determine each of the following:initial pH, pH after adding 5 mL, pH at equivalence point, pH at 11.3 mL added base, and volume of added base required to reach equivalence point

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:00
Ineed to find the answer of this question because i dont understand it
Answers: 1

Chemistry, 22.06.2019 11:00
The diagram below shows the different phase transitions that occur in matter. which arrow represents the transition in which dew is formed?
Answers: 1

Chemistry, 22.06.2019 12:30
If 22.5 liters of oxygen reacted with excess of hydrogen, how many liters of water vapor could be produced?
Answers: 3

Chemistry, 22.06.2019 17:30
To find the enthalpy of a reaction in the lab, you measured the of the reactants and the change during the reaction.
Answers: 1
You know the right answer?
Consider the titration of a 33.0 mL sample of 0.180 M HBr with 0.205 M KOH. Determine each of the fo...
Questions

Mathematics, 11.01.2021 17:40

Mathematics, 11.01.2021 17:40

Mathematics, 11.01.2021 17:40

Mathematics, 11.01.2021 17:40

Mathematics, 11.01.2021 17:40

English, 11.01.2021 17:40


Chemistry, 11.01.2021 17:40

French, 11.01.2021 17:40

Mathematics, 11.01.2021 17:40

Mathematics, 11.01.2021 17:40

English, 11.01.2021 17:40




History, 11.01.2021 17:40

Mathematics, 11.01.2021 17:40

History, 11.01.2021 17:40