
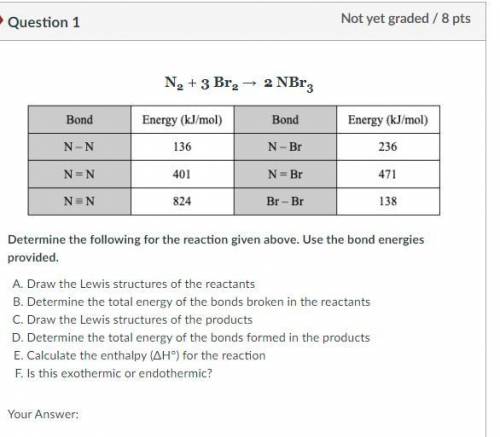
Draw the Lewis structures of the reactants
Determine the total energy of the bonds broken in the reactants
Draw the Lewis structures of the products
Determine the total energy of the bonds formed in the products
Calculate the enthalpy (ΔH°) for the reaction
Is this exothermic or endothermic?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 18:10
Consider the following reaction at equilibrium: c(s)+h2o(g)⇌co(g)+h2(g) predict whether the reaction will shift left, shift right, or remain unchanged upon each of the following disturbances. a) c is added to the reaction mixture. b) h2ois condensed and removed from the reaction mixture c) co is added to the reaction mixture d) h2 is removed from the reaction mixture.
Answers: 3

Chemistry, 22.06.2019 20:20
Which symbol can be used to indicate the pressure at which a chemical reaction is carried out? 25°c 2 atm pa
Answers: 2

Chemistry, 22.06.2019 21:30
Under which circumstances are kp and kc equal for the reaction aa(g)+bb(g)⇌cc(g)+dd(g)?
Answers: 2

Chemistry, 23.06.2019 01:00
Substance 33°f 100°f peanut oil solid liquid margarine solid liquid chocolate chips solid liquid which conclusion fits the data in the table? a. heat chemically changes chocolate and margarine. b. all solids become liquid at 100°f. c. removing heat from a substance it to melt. d. matter may change shape when it is heated.
Answers: 1
You know the right answer?
Draw the Lewis structures of the reactants
Determine the total energy of the bonds broken in the r...
Questions

History, 29.07.2019 23:30



Biology, 29.07.2019 23:30



English, 29.07.2019 23:30




History, 29.07.2019 23:30




Geography, 29.07.2019 23:30





Mathematics, 29.07.2019 23:30



