
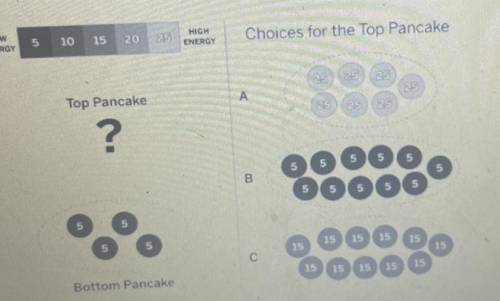
Rowan is at an all-you-can-eat breakfast. He has a pancake on his plate and is going to put another pancake on top of it. Which one of these other pancakes would make the bottom pancake the warmest? Explain how the energy and temperature of both the bottom pancake and your chosen top pancake will change after they've been touching for a while, and why.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 07:40
21. consider the following chemical reaction: n2+ o2 2 no if 10.0 g of n2 reacts with excess oxygen then how many grams of no can be formed? a) 10.7 g b) 21.4 g c) 32.9 g d) 42.8 g page 4 of 8
Answers: 2

Chemistry, 22.06.2019 10:00
Part 1: include important facts found through your research. part 2: include your visual display. include your summary of “the chemistry of water” from the national science foundation website. include your experiment. part 3: include responses to the reflection questions.
Answers: 1

Chemistry, 22.06.2019 10:40
Ammonia and oxygen react to form nitrogen monoxide and water, like this: 4nh3 (g) + 5o2 (g) → 4no (g) + 6h2o (g) also, a chemist finds that at a certain temperature the equilibrium mixture of ammonia, oxygen, nitrogen monoxide, and water has the following composition: compound pressure at equilibrium nh3 65.1atm o2 31.3atm no 62.7atm h2o 65.8atm compound pressure at equilibrium nh3 65.3 atm o2 7.79 atm no 12.1 atm h2o 65.8 atm calculate the value of the equilibrium constant kp for this reaction. round your answer to 2 significant
Answers: 2
You know the right answer?
Rowan is at an all-you-can-eat breakfast. He has a pancake on his plate and is going to put another...
Questions

Mathematics, 16.08.2019 11:10



Social Studies, 16.08.2019 11:10



Mathematics, 16.08.2019 13:10

Biology, 16.08.2019 13:10

Biology, 16.08.2019 13:10





Mathematics, 16.08.2019 13:10


Mathematics, 16.08.2019 13:10






