
Chemistry, 05.05.2021 01:00 rachelsweeney10
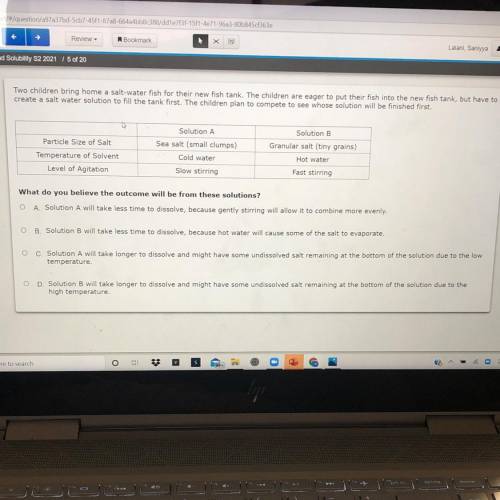
Two children bring home a salt-water fish for their new fish tank. The children are eager to put their fish into the new fish tank, but have to
create a salt water solution to fill the tank first. The children plan to compete to see whose solution will be finished first.
Particle Size of Salt
Temperature of Solvent
Level of Agitation
Solution A
Sea salt (small clumps)
Cold water
Slow stirring
Solution B
Granular salt (tiny grains)
Hot water
Fast stirring
What do you believe the outcome will be from these solutions?
O A Solution A will take less time to dissolve, because gently stirring will allow it to combine more evenly
OB Solution will take less time to dissolve, because hot water will cause some of the salt to evaporate.
O c Solution A will take longer to dissolve and might have some undissolved salt remaining at the bottom of the solution due to the low
temperature.
OD Solution will take longer to dissolve and might have some undissolved salt remaining at the bottom of the solution due to the
high temperature

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:30
Which orbitals form a pi bond? a.the s orbital and three p orbitals b.the s orbital and two p orbitals c.overlapping p orbitals d.overlapping hybrid orbitals
Answers: 2

Chemistry, 22.06.2019 00:10
According to the diagram; a) identify the anode of the cell and write the half-reaction that occurs there b) write the overall equation for the reaction that occurs as the cell operates c) calculate the value of the standard cell potential ,e cell. d)write the shorthand notation of the cell above e)indicate the flow of the electrons on the diagram
Answers: 3

Chemistry, 22.06.2019 00:30
13. calculate the initial concentration (before precipitation) of carbonate ions after the addition of each 0.05 ml of solution b to the 1.00 l beaker of solution a. divide the work among group members and write the answers in the table in model 3. assume the volume change as solution b is added is negligible. 14. notice the initial concentrations of zn2+ - and cu2+ in the table in model 3. a. explain how these were obtained from the data in model 2. b. as solution b is added and precipitates form, do these initial concentrations change? 15. use the data in model 2 to indicate the presence of precipitate (either znco3 or cuco3) after each 0.05 ml addition of solution b in model 3. 16. use the initial concentrations of carbonate ions and zinc ions to calculate the reaction quotient, qsp for the zinc carbonate scenarios in model 3. divide the work among group members and write the answers in the table in model 3. 17. use the initial concentrations of carbonate ion and copper(ii) ions to calculate the qsp for the copper(ii) carbonate scenarios in model 3. divide the work among group members and write the answers in the table in model 3.
Answers: 3

You know the right answer?
Two children bring home a salt-water fish for their new fish tank. The children are eager to put the...
Questions


Mathematics, 26.05.2021 22:30

History, 26.05.2021 22:30

Mathematics, 26.05.2021 22:30

Geography, 26.05.2021 22:30



History, 26.05.2021 22:30

Mathematics, 26.05.2021 22:30

Mathematics, 26.05.2021 22:30

Chemistry, 26.05.2021 22:30

Chemistry, 26.05.2021 22:30



Mathematics, 26.05.2021 22:30

English, 26.05.2021 22:30

Medicine, 26.05.2021 22:30

Chemistry, 26.05.2021 22:30

Mathematics, 26.05.2021 22:30

Advanced Placement (AP), 26.05.2021 22:30



