
Use the References to access important values if needed for this question.
Please note that "geometry" refers to the molecular or ionic geometry.
In the VSEPR model, the geometry of the regions defined by the electron pairs in the valence shell of an atom is called the electron-pair geometry.
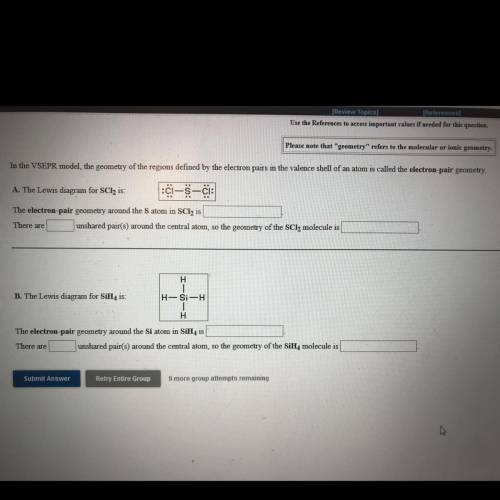
A. The Lewis diagram for SCl2 is: C-5-c:
The electron-pair geometry around the Satom in SCl2 is
There are
unshared pair(s) around the central atom, so the geometry of the SCl2 molecule is
H
B. The Lewis diagram for SiH, is:
H-Si-H
H
The electron-pair geometry around the Si atom in SiH, is
There are unshared pair(s) around the central atom, so the geometry of the SiH, molecule is
Submit Answer
Retry Entire Group
9 more group attempts remaining

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:30
The clouds are grey and ground is wet. a quantitative b qualitative
Answers: 1


Chemistry, 22.06.2019 09:00
Chen drew a diagram to compare the ways in which different organisms obtain nitrogen. which label belongs to the area marked z?
Answers: 3

You know the right answer?
Use the References to access important values if needed for this question.
Please note that "geome...
Questions

English, 23.12.2019 22:31






Biology, 23.12.2019 22:31




Social Studies, 23.12.2019 22:31



Social Studies, 23.12.2019 22:31

Biology, 23.12.2019 22:31



Mathematics, 23.12.2019 22:31




