
Chemistry, 05.05.2021 02:10 peachijmin
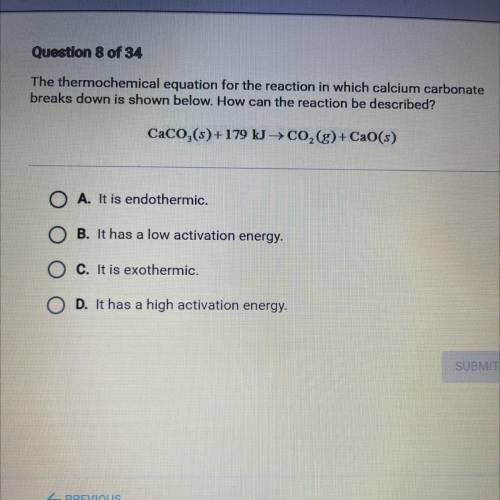
The thermochemical equation for the reaction in which calcium carbonate
breaks down is shown below. How can the reaction be described?
CaCO,(s)+179 kg CO,(g)+ CaO(s)
A. It is endothermic.
B. It has a low activation energy.
C. It is exothermic.
D. It has a high activation energy.
NO LINKS

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:30
Which mathematical relationship should you us to convert moles of a substance into grams
Answers: 1

Chemistry, 22.06.2019 12:30
The melting point of sulfur is 115 °c and its boiling point is 445 °c. what state would sulfur be in at 200 °c?
Answers: 1

Chemistry, 22.06.2019 13:30
Mary is conducting an experiment on how pollution affects plant growth. how can she ensure that her data are reliable?
Answers: 3

Chemistry, 22.06.2019 14:30
Select all that apply. using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 (s) pb+2(aq) + 2cl -(aq). the concentration of the products yield a ksp of 2.1 x 10-2:
Answers: 2
You know the right answer?
The thermochemical equation for the reaction in which calcium carbonate
breaks down is shown below...
Questions

Mathematics, 22.01.2022 20:10






Mathematics, 22.01.2022 20:20


Mathematics, 22.01.2022 20:20


Mathematics, 22.01.2022 20:20



Mathematics, 22.01.2022 20:20


Mathematics, 22.01.2022 20:20

Computers and Technology, 22.01.2022 20:20


Mathematics, 22.01.2022 20:20

Mathematics, 22.01.2022 20:20



