
Chemistry, 05.05.2021 08:40 EllaLovesAnime
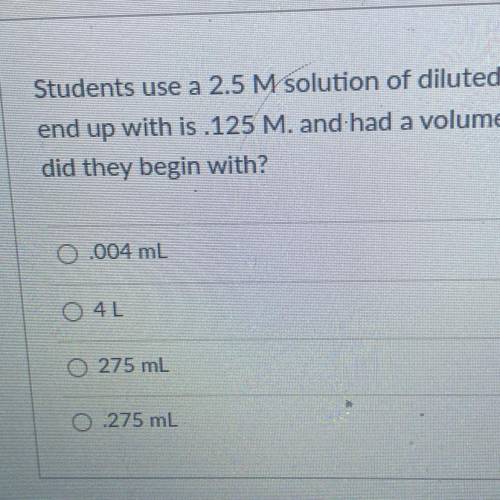
Students use a 2.5 M solution of diluted hydrochloric acid. The Molarity of the solution they
end up with is 125 M. and had a volume of 5500mL. What volume of the concentrated HCI
did they begin with?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Which of the following two events occur to create a sea breeze? select all that apply. warm air rises on the ocean and moves toward the land to cool warm air rises on land and moves toward the ocean to cool cool air moves from the ocean to be warmed by the land cool air moves from the land to be warmed by the ocean
Answers: 3

Chemistry, 22.06.2019 09:40
Sulfur dioxide and oxygen react to form sulfur trioxide during one of the key steps in sulfuric acid synthesis. an industrial chemist studying this reaction fills a 25.0l tank with 4.5 mol of sulfur dioxide gas and 4.5 mol of oxygen gas at 30.°c. he then raises the temperature, and when the mixture has come to equilibrium measures the amount of sulfur trioxide gas to be 1.4 mol. calculate the concentration equilibrium constant for the reaction of sulfur dioxide and oxygen at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 3

Chemistry, 22.06.2019 12:00
What is the lowest number energy level where a d sublevel is found
Answers: 1

Chemistry, 22.06.2019 22:30
What if it is did darwin used to support his theory of evolution
Answers: 1
You know the right answer?
Students use a 2.5 M solution of diluted hydrochloric acid. The Molarity of the solution they
end...
Questions

History, 18.04.2020 04:13


Biology, 18.04.2020 04:13

Mathematics, 18.04.2020 04:14



Health, 18.04.2020 04:14







Mathematics, 18.04.2020 04:14




Computers and Technology, 18.04.2020 04:14


English, 18.04.2020 04:14



