
Chemistry, 05.05.2021 23:20 bryanmcmillianjr
Calculate the Gibbs free energy change in kJ/mol at 25.0 C for the reaction: C3H8(g) + 5O2(g) ---> 3CO2(g) + 4H2O(g)
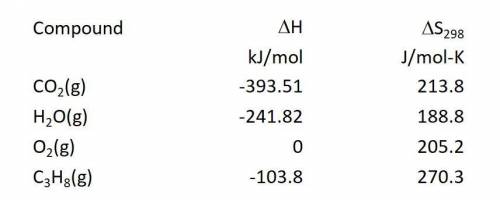

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Complete the sentence. the lower the hydrogen ion concentration, the the ph. higher lower closer to 7 closer to 0
Answers: 2

Chemistry, 22.06.2019 13:30
Mary is conducting an experiment on how pollution affects plant growth. how can she ensure that her data are reliable?
Answers: 3

Chemistry, 22.06.2019 16:10
Given the following equation: 2a1 + 3mgcl2 --> 2alcl3 + 3mg how many moles of aluminum chloride are produced from 2.5 moles of magnesium chloride?
Answers: 1

Chemistry, 22.06.2019 16:30
How many grams of mgbr2 are needed to produce 75g or metal?
Answers: 1
You know the right answer?
Calculate the Gibbs free energy change in kJ/mol at 25.0 C for the reaction: C3H8(g) + 5O2(g) --->...
Questions

Geography, 19.09.2019 14:30

History, 19.09.2019 14:30


History, 19.09.2019 14:30

Advanced Placement (AP), 19.09.2019 14:30




Biology, 19.09.2019 14:30



Biology, 19.09.2019 14:30


History, 19.09.2019 14:30



Mathematics, 19.09.2019 14:30


Mathematics, 19.09.2019 14:30




