
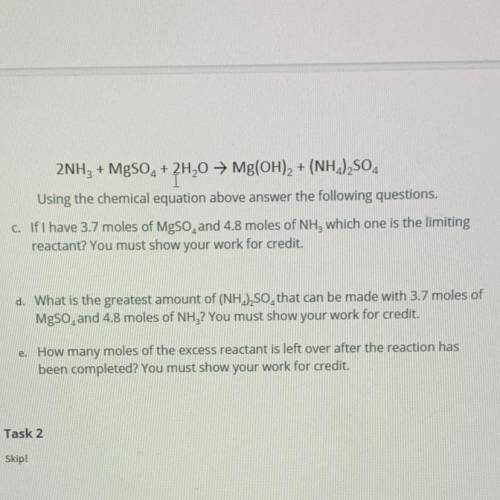
2NH3 + MgSO2 + 2H20 → Mg(OH)2 + (NH2)2SO4
Using the chemical equation above answer the following questions.
c. If I have 3.7 moles of MgSO, and 4.8 moles of NH, which one is the limiting
reactant? You must show your work for credit.
d. What is the greatest amount of (NH.),SO, that can be made with 3.7 moles of
MgSO, and 4.8 moles of NH3? You must show your work for credit.
e. How many moles of the excess reactant is left over after the reaction has
been completed? You must show your work for credit.
Please show work it would be greatly appreciated

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:20
What would you do if you told the guy you liked that you liked him
Answers: 1

Chemistry, 22.06.2019 11:40
Enzymes affect the reactions in living cells by changing the
Answers: 3

Chemistry, 22.06.2019 18:00
Which statement best describes the he properties of iconic compounds ?
Answers: 1

You know the right answer?
2NH3 + MgSO2 + 2H20 → Mg(OH)2 + (NH2)2SO4
Using the chemical equation above answer the following q...
Questions

Mathematics, 25.02.2021 04:30

Mathematics, 25.02.2021 04:30


Mathematics, 25.02.2021 04:30


Mathematics, 25.02.2021 04:30

Biology, 25.02.2021 04:30

History, 25.02.2021 04:30




Chemistry, 25.02.2021 04:30

History, 25.02.2021 04:30


English, 25.02.2021 04:30

English, 25.02.2021 04:30



Mathematics, 25.02.2021 04:30

Health, 25.02.2021 04:30



